[Trade Journal]
Publication: Metallurgical and Chemical Engineering
New York, NY, United States
vol. 16, no. 8, p. 433-436, col. 1-2
Electrical Porcelain
By L. E. Barringer
President, American Ceramic Society
In joining this discussion concerning ceramic products I wish to first take advantage of such a gathering of chemists and engineers to explain the term "ceramics," as now understood by those immediately interested or engaged in ceramic engineering. Originally the term referred to strictly art wares such as might be found in museums or in Fifth Avenue shops, but during the past few years has been applied to the industrial and technical field concerned with the use or production of silicates. There are, of course, exceptions as in the making of magnesia brick in which no silicate is employed, or in the production of certain cements, but in general the ceramic industry may be defined as I have just indicated.
The three main classes of the ceramic industry are the clay, glass and cement industries and a smaller but very important branch is that of enameling metal wares for cooking, etc.
In the clay and cement industries use is made of silicates as raw materials.
In the glass and enamel industries silicates are produced from oxides, carbonates, feldspars, silica, etc.
There is no industry which can be more sharply or consistently defined than the ceramic industry and I am very anxious to see the term more generally and clearly understood among the engineering profession in general other than those immediately interested in ceramics.
CERAMIC INSULATION
In the electrical industry ceramic products (silicates), are employed extensively as insulating materials and I need only mention such substances as mica, slate, soap stone, glass, porcelain and asbestos products to indicate immediately the extensive and varied use to which silicate or ceramic products are applied in the manufacture of electrical apparatus.
PORCELAIN FOR ELECTRICAL PURPOSES
This evening I am dealing with only one of these ceramic insulating products but probably the most important with the exception of mica, namely, electrical porcelain.
The art of making porcelain is practically as "old as the hills" and originated in China in very early times. The history of the development and progress of this highest of ceramic products is interwoven with the history of the world.
It was not until about 1852, however, that the use of porcelain was developed as electrical insulation. At that time attempts were being made to string telephone wires using gutta percha insulators. It was found that such material was not weather-resisting, however, and also that the sulphur mixed with the gutta percha rapidly corroded the copper wires. In experimenting it was found that adding clay to the mixture gave very much better results and that the more clay there was added the better the insulation. This logically led to using practically all clay and crude clay insulators were finally adopted. The gradual progress from such crude clay insulators to porcelain or the highest grade of clay insulator was natural and porcelain was finally employed for purposes of insulating overhead lines and any other places where a high-grade weather-resistant insulation was required.
Porcelain is preferable to and distinguished from other clay wares by possessing to a pre-eminent degree the properties of hardness, whiteness, high fusibility, translucency and density or impermeability.
Porcelain has a high dielectric strength and insulation resistance and a 1/4-in. thickness will usually not puncture under 70,000 volts and a 1/2-in. thickness under 100,000 volts. The increase of dielectric strength with thickness follows roughly a quadratic curve. As in all other insulating materials, both the dielectric strength and the insulation resistance fall with rise of temperature and at 300 deg. C. porcelain becomes a very poor insulator indeed. In fact, the dielectric strength begins to fall at 100 deg. C. and decreases very rapidly until the insulation is of a small order at 300 deg. C. The decrease of insulation resistance with rise of temperature is of the order 100 to 1 with rise of 51 deg. C.
The specific gravity of electrical porcelain is 2.4 and the coefficient of expansion 0.000004 per degree C. This coefficient of expansion is about one-third that of steel, one-quarter that of copper and one-fifth that of brass.
Porcelain is waterproof, weather-resistant, not attacked by oils, gases or vapors, shows no warping or shrinking with age, is readily formed or molded into the various intricate shapes required, is of comparatively low cost and has a pleasing appearance.
The crushing strength is high, averaging 20,000 lb. per square inch and the tensile strength ranges from 900 to 1800 per square inch.
From what I have said so far it might seem that porcelain was a splendid all-around insulating material and that there would be little or no necessity for the many other insulating materials which are employed in the electrical industry; but there are certain deficiencies in porcelain which prevent it from being the ideal insulation for all purposes.
In the first place, there is a lack of toughness in porcelain such as is required to withstand sudden shock or impact, or even the jar or vibration of certain pieces of apparatus. In using porcelain insulators on battle ships, for instance, it has been found that the gun fire will cause excessive breakage. The same is also true with reference to using porcelain insulators on electric locomotives or electric cars, where there is a considerable amount of jar or vibration. In electrical porcelain, there is also little or no resistance to sudden heating or cooling and for this reason the material would not be suitable for use in controllers or rheostats or in other places where there is intermittent heating and cooling. It might be asked why electrical porcelain does not stand heating and cooling as well as chemical porcelain, but I would call your attention to the great difference in design. The thin and uniform walls of porcelain dishes used in the laboratory permit of their being heated through uniformly and quickly so that there is no great expansion differential. With the large irregular shaped porcelains used in high-voltage insulation, however, any sudden heating causes local expansion and gives rise to cracking. The heat conductivity of porcelain is very low and a thick-walled piece will not heat through quickly.
There is also the drawback of non-flexibility and porcelain cannot be used for such purposes as insulating armature coils or other moving elements of electrical machinery where there is considerable bending and torsional strain.
Finally, metal parts cannot be imbedded or molded into place in porcelain as the high temperature used in burning will either melt the metals or else flux them with the silica of the porcelain mixture. In certain cases of insulation, therefore, the rigidity of construction cannot be secured with porcelain as with certain other molded materials.
The composition and method of manufacture of electrical porcelain is not unlike that of other porcelain wares, as ornamental porcelain, chemical porcelain, etc. The materials employed in producing high-grade porcelain are kaolin, or china clay, ball clay, feldspar and silica.
The clays possess the valuable element of plasticity which permits of the ware being readily formed into the various shapes required. The clays have also the very valuable property of becoming indestructible upon firing. When clays are used alone, however, a porous mass or "body" is secured when the wares are fired unless the heat is carried to excessive temperatures which quickly melt or distort the pieces.
Feldspar is used as a flux to give a dense, vitrified body at a temperature readily attainable.
Flint is used as a stabilizer to extend the vitrifying range and to prevent the distortion of the pieces within a narrow temperature range as would result were feldspar and clay used alone.
All are familiar with the chemical changes which take place upon heating such a mixture as I have described. As the heat in the kiln increases the clay loses its combined water, this occurring within a temperature range of 600 to 900 deg. C. In the neighborhood of 1200 deg. C. the feldspar fuses and the clay begins to dissociate with the formation of sillimanite (A12O3, SiO2). As the temperature still further increases the quartz is slowly attacked or dissolved by the feldspar up to 1400 deg. C., when solution is practically complete.
The final product consists of glass or fused feldspar in which is dissolved quartz and the silica resulting from the dissociation of the clay, sillimanite crystals and more or less of residual or undissolved quartz, depending upon the final temperature, or degree of vitrification.
Professor Binns has already told of the general processes by which porcelain is manufactured and I will not repeat what he has said. For electrical porcelain the mixtures are prepared and handled practically along the same lines as has just been described by Professor Binns.
| |||
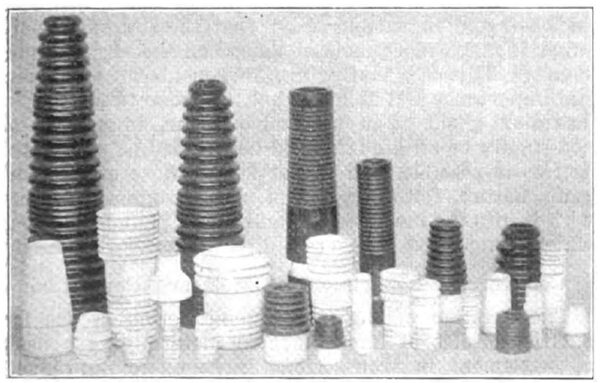
| Fig. 2 -- Wet-Prcess Porcelain Insulation |
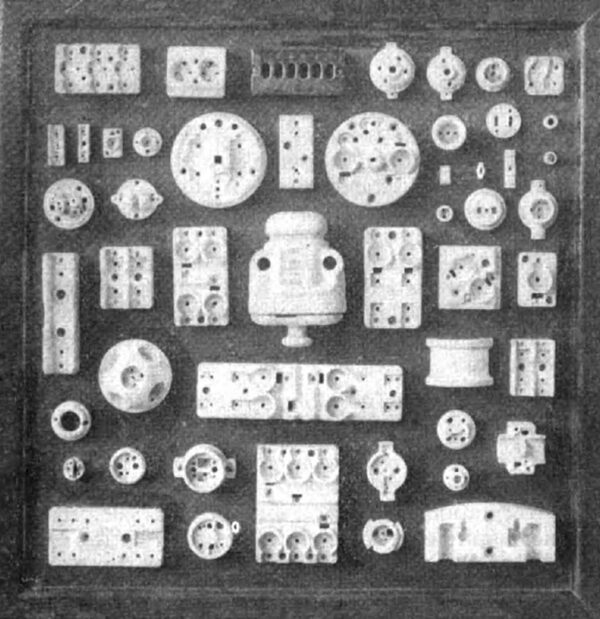
In the manufacture of electrical porcelain, however, the ware is classified into dry and wet processes, the former being that class of ware which is formed in steel dies and the latter that class of ware which is formed from the plastic porcelain mixture in the same manner as chemical or art porcelain. Figs. 1 and 2 illustrate both these classes of porcelain. The dry-process porcelain is used for low-voltage insulation and is seen in the form of cleats and knobs, etc., for the wiring of buildings for electric lighting. Wet-process porcelain is used for the making of insulators for high-voltage apparatus, as transformers, and high-tension switches and as insulators for high-voltage transmission lines. The wet process porcelain is of considerably higher dielectric strength than the dry-process and is not absorbent. Wet process porcelain will not absorb over 1/4 of 1 per cent of water when immersed for forty-eight hours, whereas the dry-process may absorb as high as 1 per cent under the same conditions. The two classes of electrical porcelain are the same in composition but the difference in porosity and dielectric strength results from the different methods used in preparing the mixture.
| |||
| Fig. 1 -- Dry Process Porcelain Insulation |
In the drying and burning of electrical porcelain there is no fundamental difference between the operations and the same operations in the handling of chemicals and art porcelains, and Professor Binns has already explained to you the general method of procedure.
I wish to call your particular attention, however, to an advanced type of kiln for firing porcelain. For many years the old bottle-shaped potter's kiln was used and all are no doubt familiar with this type of kiln. It produced the desired results but was extremely wasteful of heat. During the past few years types of continuous kilns have been devised and we are now operating a continuous kiln at Schenectady with very successful results and are saving about 50 per cent of the fuel required for operating the up-draft potter's kiln.
| |||
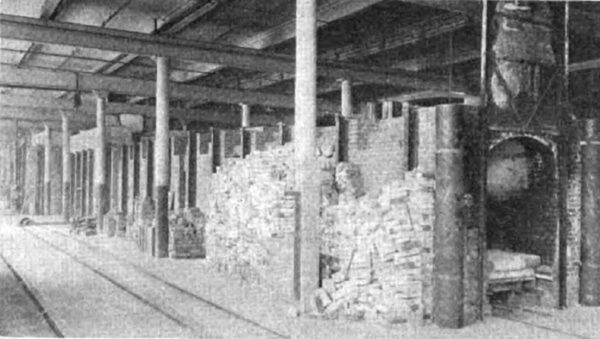
| Fig. 3 -- Continuous Kiln From Discharge End |
| |||
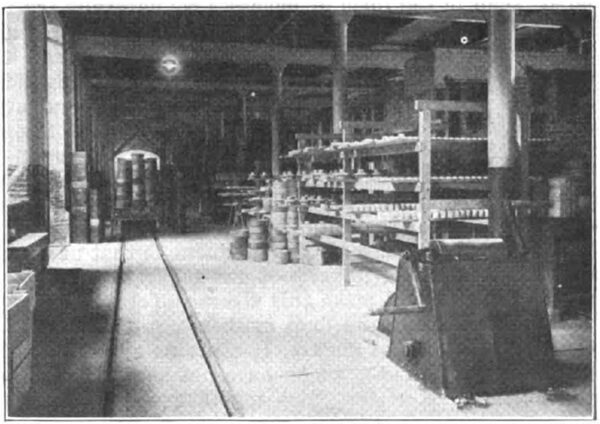
| Fig. 4 -- Side View, Continuous Kiln |
The kiln (Figs. 3 and 4) is a straight tunnel, 197 ft. long, with a fixed firing zone somewhat nearer the discharging end than the charging end. The ware is placed upon cars and moved through the firing zone to the cooling end. At the firing zone there are grates on either side. This combustion chamber is separated from the main tunnel by a narrow wall and the gases passed through tuyeres before entering the main tunnel. Air for combustion is preheated by passing through port holes at the discharge end. The air is heated by coming in contact with the outgoing cars of finished and cooling ware. Just before reaching the firing zone the flue is divided and part of the air (primary air), is deflected to beneath the grates to be used for combustion of the fuel. A second flue deflects a part of the preheated air (secondary air), into the main tunnel at the firing zone area where it serves for the combustion of incompletely consumed gases from the fire boxes. The gaseous products of combustion pass toward the receiving end of the kiln and serve to heat up the incoming cars of green or cold ware and then pass into the main stack flue at the receiving end of the kiln. The stack temperature is only from 200 to 220 deg. C.
The temperature of burning is 1300 to 1400 deg. C. All ware is fired in saggers and a sand-seal protects the running gear of the cars from the burning gases.
Each car carries about 87 saggers 12 in. diameter of 6-in. high (inside). A kiln of the type under discussion is equivalent to about 3-1/2 of the ordinary potters' kilns of 14 ft. diameter. In addition to a saving in coal of about 50 per cent it has been found that the labor of handling or manipulating the kiln is slightly less and that the life of the saggers is increased.
This kiln represents a radical change and improvement in the method of firing, not only electrical porcelain, but ceramic wares of all kinds. General Electric Co., Schenectady, N. Y.
*An address made at the Feb. 9, 1917, meeting of the New York Section of the American Electrochemical Society, in joint session with the New York Section of the American Chemical Society and the Society of Chemical Industry.