[Trade Journal]
Publication: The Telegrapher
New York, NY, United States
vol. 8, no. 60, p. 473, col. 1-3
Every Day Problems in Practical Telegraphy.
By F. L. POPE.
NOTWITHSTANDING the great advance which has taken place within the last few years in respect to what may properly be termed the science of telegraphy, yet it is an undeniable fact that we still find many time-honored and popular electrical fallacies, which, having once become securely fixed in the mind of the average telegraph operator or superintendent, seem to bid defiance to all efforts to dislodge them.
I was quite forcibly reminded of the above mentioned circumstance on a recent occasion, in conversation with a very worthy and excellent line man - one who takes a just pride in the neatness and thoroughness with which he does his work. He had a few weeks before finished a job of running a large number of wires - perhaps 25 or 30 - into a new office. The line wires, all of No. 8 gauge, were brought down from a single pole, perhaps 50 feet from the building, carried underneath the roof of a broad overhanging verandah, where they turned at a right angle and entered the building through hard rubber tubes over the windows. The office wires were connected to the line wires inside the office. The whole of the outside work had been done with No. 8 iron wire and glass and pin insulators, and well and carefully done, too; but a few weeks had been sufficient to develop some very prominent defects. The short sections of heavy wire would, of course, only remain taut when strained to the utmost practicable tension. The turning of the insulators upon the wooden pins, and the giving way of the pins themselves under the strain at the angle, although slight, had been sufficient, in connection with the yielding of the pole, to allow the wires to slacken. This, of course, caused numerous incipient kinks and bends to appear, and utterly spoiled the looks of the job, to say nothing of giving rise to well founded apprehensions of future crosses.
My friend, the line man, naturally felt a little troubled at this result, after he had taken so much pains with the work, but remarked, with much show of reason, that no One else could do it any better with No. 8 wire and glass and pin insulation. With No. 12 wire, and bard rubber suspension insulators, he thought he could have made a handsome and permanent job. But the superintendent would not allow this, alleging that the No. 12 wire " put too much resistance in the line," and that the kind of insulation mentioned above "was not as good as glass."
If we examine this matter a little we shall find a good specimen of a current telegraphic fallacy, which no one seems to have thought worth his while either to prove or disprove. I have even beard it asserted, by persons laying claims to superior telegraphic knowledge, that in the case mentioned the capacity of the whole line would be reduced to that of a No: 12 wire!
Suppose we allow 100 feet of wire, from the pole to the office window, for each line. This would be 200 feet (in and out) in each through circuit. If we estimate the resistance of the No. 8 wire Ľat 16 units per mile, and that of No. 12 at 36 units per mile, which is about the average, we find the resistance of 200 feet of each kind to be respectively 0.4 units and 1.3 units. The increase in the resistance of the line, caused by the substitution of the small wire would, therefore, amount to precisely nine tenths of a unit! Au ordinary telegraph line of 150 miles, in good condition, usually gives, including relays in circuit, from 4,000 to 6,000 units resistance. The damage resulting from the addition of less than one unit to the above amount is certainly largely overestimated in the popular mind! Even if this amount of small wire were inserted at each of 20 stations the effect upon the circuit would not be perceptible, being scarcely more than one tenth of the additional resistance caused by the insertion of a single relay. The real objection to the use of small wire is confined to cities and localities exposed to smoke and corrosive gases, where a small wire is very quickly eaten away by oxidization, if not protected by a coating of paint or tar.
In respect to insulation the case is much the same. Suppose the common rubber insulator was three times as bad as the glass and pin "(which it isn't), and it required six of them to run 'the wire in and out, the escape from the six would in that case be equal to that from 18 glass insulators, an increase in the escape equal to the addition of 12 glass insulators. On a line of 150 miles this would increase the escape about 1/500! But the Brooks or improved rubber might be used in place of the old rubber, in which case the loss would be below computation.
"The bearings of the above observations lies in the application thereof." If it is necessary to use a No. 12 wire, in reasonable amount, to make a handsome and permanent job in running into an office, no one need hesitate to do so for fear of any practical ill effect arising therefrom. Of course, if the amount of small wire inserted is measured by miles instead of feet, there will be a perceptible diminution in the strength of the circuit.
I was once called upon to prescribe for a certain "short wire," which, although it had been put up a considerable length of time, I was informed had never worked satisfactorily, or indeed at all, except upon rare occasions. All the telegraphic talent in the vicinity had endeavored to make it work, but thus far without success. The line was three miles long, and had five offices on it, and was supposed to be worked by a carbon battery of seven cups. I suspected the ground connections were faulty, and found upon examination that one of them consisted of an iron rod driven three or four feet into a gravelly soil, and the other, a piece of sheet iron, of perhaps ten square feet, buried in tolerably damp sand, resting on a bed of gravel. This looked well enough to all appearances, but the application of a galvanometer told a very different story, and at once revealed the source of the trouble. I give the measurement from my note book:
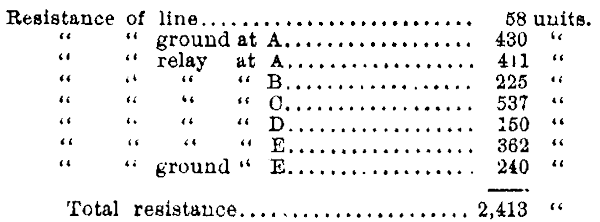 |
This is equivalent to a good line of 100 miles, with five ordinary relays, of 150 units resistance each. It is not much to be wondered at that it failed to work well with seven cups of battery, especially in a dry season,
If it had been possible in this case to make good ground connections with gas and water pipes at each end, and substituted relays prepared expressly for the line, of say twelve units each, the total resistance would have been about as follows:
 |
Such a line and instruments would work well on one cup of carbon battery, and very strong on two. The best that could be done, however, in the case mentioned was to get a ground on a pump at one end, and put the plate in a stream of water at the other. The line was then equipped with relays of 150 units. The seven cups of carbon worked it very well indeed after these changes were made.
The moral of the above narrative is, that a galvanometer is a very handy implement to have about the house, especially in localities where Ohm's laws are in force.
A friend of mine, who has charge of a railroad line on which the Callaud battery is used for the main circuits, complained that much trouble was experienced from the connecting wires being eaten off by the action of the battery. As this is not at all an uncommon circumstance, it occurred to me that it might be advisable to point out the cause of the difficulty. In this instance the copper was in the form of a cup two inches deep, nearly filling the bottom of the jar. The connecting wire was soldered to the perpendicular side of the cup, and in most cases the gutta percha had been removed for a quarter of an inch or, so above the upper edge of the cup. Occasionally the division line between the copper and zinc solutions would, stand just at the top of the copper cup. This formed a local' galvanic circuit, consisting of two dissimilar liquids and one metal, by means of which the latter was soon destroyed at that point. The remedy is to attach the wire at the bottom of the copper plate, and have the gutta-percha protect it all the way down to the lowest point. Copper wires sometimes break off at the point where they are soldered, owing to the fact that this metal when heated and allowed to cool slowly becomes brittle. Its action in this respect seems to be, curiously enough, the exact reverse of that of iron.
When oil is used on the surface of a Callaud battery to prevent evaporation, it combines with the deposit of black oxide on the zincs when the latter are taken out, and makes it a disagreeable and troublesome job to clean them. If the zinc are dipped for a few seconds in a solution of caustic (washing) soda and water, after being taken out of the battery, the deposit comes off by the aid of a common battery brush with the greatest ease, leaving the zincs perfectly clean. The same solution is useful to apply to joints after soldering, as it neutralizes all the remaining acid, which would otherwise corrode the wires to a serious extent.
It is a great convenience, in using the Calland battery, to have it placed so that its internal condition can be seen without difficulty. Mr. John Suter, division operator of the Pennsylvania R. R. at Pittsburg - who, by the way, is one of the most sensible and practical telegraph men I have ever met - places his Callaud locals in a case, with shelves and glass doors, on the wall of the office, about four or five feet from the floor. One great advantage in this arrangement is that the batteries are far less likely to be neglected by the operators when in plain sight than when stowed away in a box in some out of the way corner. Moreover, a battery kept neat and clean, and enclosed in a handsome ease, is decidedly an ornamental addition to the fixtures of an office.
