[Trade Journal]
Publication: Glass Industry
New York, NY, United States
vol. 7, no. 6, p. 136, col. 1-2
New Glass Transmits Ultra-Violet Rays
By Fred M. Locke
A new glass for transmitting ultra-violet rays, which is a radical departure in composition from any of the different kinds of glass now upon the market has been developed and patented¹ by Fred M. Locke Research Laboratory.
It is well known that ordinary plate and window glass and other glasses absorb a greater part of the ultra-violet rays, and that below a wavelength of 300 Mu they do not transmit any appreciable percentage. It is, however, below a wavelength of 300 Mu, down to 270 Mu that the greatest healing power of these rays is found. However, up to the present no glass has been found, with exception of fused quartz, that will transmit at these wavelengths. As is well known, the cost of fused quartz is prohibitive for most uses.
This new glass when in relatively thin pieces, will equal fused quartz in its transmission, transmitting as low as 200 Mu and in pieces up to one-half inch thick will transmit far into the ultra-violet.
The cost of this new glass is only slightly higher than ordinary plate or window glass. It can be melted in the ordinary tank or pot, and is exceptionally clear and transparent, equal to that of the best optical glass.
It has also a number of other interesting characteristics, one being its low expansion, about that of the best chemical glass i.e., 0.0000035. It also has the property of insulating electricity at a high temperature, up to the melting point of the glass itself.
In its simpler form this glass contains only four ingredients and is substantially free from the alkalies, soda, potash and lithia.
The following table gives number of raw batches which will produce this glass:
 |
In the following table the glasses A to H inclusive are glasses calculated from the batches 1 to 8, inclusive, of the foregoing table. The glass I is an additional glass of a similar nature:
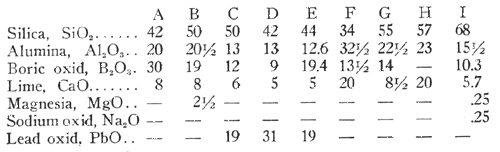 |
All of the above glasses have a low silica content, not exceeding 70 per cent and this content may be reduced as low as 25 per cent to obtain some of the properties of this type of glass.
It will be noted that all the batches as well as the glasses contain large amounts of alumina. In the examples given, the finished glasses contain from 12.6% to 32.5% of alumina. However, it has been found that some of the properties of these glasses may be secured by providing an alumina content of 10% to 35% in the finished glass. Alumina has not heretofore been used in low-expansion glasses in large amounts, because when combined with an alkali it will produce large expansion or make the glass so hard that it is impracticable to work. In this type of glasses the alumina appears to act as a flux and increases the workability. It stops the danger of crystallization, increases stability, and tends to make low expansion.
It is evident that the compositions given are radical changes from the usual glasses. Heretofore the use of fluorspar in any appreciable amounts in glass was for the purpose of obtaining opaque or opal glasses, and it was known that by the use of such material in large amounts the pots or refractories used were attacked to a marked degree. It was found, however, that the use of fluorspar together with large amounts of alumina resulted in glasses that were exceptionally clear and transparent, and that the fluorspar did not attack the pot as much as would the ordinary type of alkali glasses.
Experiments have proved that the best results are obtained when using calcium fluoride as the principal fluxing material. However, other fluorides or other compounds of the alkaline earth metals may be employed, particularly if it is not desirable to obtain transparent glass.
In some of the batches the boric oxide has been partially replaced by lead for the increasing of the fusibility and workability of the batch, while at the same time the tendency to devitrification has decreased. Lead also tends to better the insulating properties of these glasses. Instead of lead barium may he used in some cases.
A further advantage of this type of glass is high stability against the attack of steam, water, acids and atmospheric conditions and a fair stability against the action of alkalies. This latter property is unusual, as glasses heretofore made with substantially no alkali content are readily attacked by alkaline solutions, especially under the action of heat.
This type of glass may be melted or worked in an ordinary pot or tank. It is plastic enough through a considerable range of temperature to allow it to be pressed or blown into shape in the ordinary manner.
While this glass was originally developed as a material for electrical insulators, spark plugs and radio power transmitting tubes, it was found that it also has the power of transmitting ultra-violet rays in large percentages. As an example of the batches shown in the above table, batch No. 1 will when made into glass about 2 millimeter in thickness, transmit as low a wavelength at 230 Mu. This glass is composed, as will be noted, of only silica, boric oxide, alumina and fluorspar. Other glasses can be made which will transmit further than this, down to 200 Mu.
*The Fred M. Locke Research Laboratory, Victor, N. Y.
¹U. S. Patent No. 1,529,259
