[Trade Journal]
Publication: The Telegrapher
New York, NY, United States
vol. III, no. 50, p. 177-178, col. 1-3, 1-2
Comparative Tests of Telegraph Insulators.
OFFICE SUPT. POLICE AND FIRE ALARM TEL.,
N. W. COR. FIFTH AND CHESTNUT STS.,
PHILADELPHIA, JAN. 30, 1867.
Dr. C. M. CRESSON:
MY DEAR SIR: - I send herewith perfect specimens of insulators of their kind, labeled and numbered as they are known. Will you in your own way examine and report the resistance qualities of these insulators, and otherwise give such information as will help me to form an opinion of their merits?
I am, very truly yours,
W. J. Phillips,
Supt. Police and Fire Alarm Telegraph.
No. 417 WALNUT ST., PHILADELPHIA,
March 16, 1867,
WM. J. PHILLIPS, Supt. Police and Fire Alarm Tel.:
DEAR SIR: - In accordance with your request of Jan. 30, I have made experimental trials upon the comparative value of the several forms of insulators in common use upon the telegraph lines of this country and in Europe, samples of which you sent to me, and in the course of the investigation have subjected them to various tests, and made such an examination as to show wherein they differed in usefulness, and the reasons for such differences.
The requisites for a good telegraphic line insulator may be summed up as follows: It should be a non-conductor of electricity, and a non-absorbent of moisture, with a surface free from pores or cracks; it should be a bad conductor of heat, and composed of a substance not capable of being wetted, and which shall be durable, and not disintegrate or crack when subjected to changes of heat and cold, or dry or moist atmosphere; it should also be of such form as shall shed the weather, and be easily removed or replaced.
Among all the materials commonly employed as non-conductors of galvanic electricity, very little difference is observable in their conducting power when in a dry condition. Dry wood, glass, vulcanized rubber, pottery, or any substance free from the intermixture of metals or their oxides, give equally good results when dry. It is only when the great enemy of insulation - water - is present that these bodies become in a greater or less degree conductors.
Excluding those substances the pores of which are too minute to be appreciable, such as glass and hard rubber, all of the others may be tabulated in the order of their capacity for the absorption of moisture, classing those as best which take up the least weight of water when soaked for a given time, such as China-ware, pottery and wood. Next in importance it is necessary that the insulating medium be free from surface fissures into which water may be drawn or retained by capillary attraction.
To this objection all of the materials ordinarily employed are liable, even glass and hard rubber* soon yield to atmospheric influences and become filled with innumerable fine surface cracks or crevices from which it is almost impossible to remove the water otherwise than by artificial heat. The insulating body should be a bad conductor of heat, otherwise changes of temperature from cold to warm, even when unaccompanied by rain or fog, would cause a deposition of moisture upon the insulator, including that portion of the surface which from peculiarity of form is protected from the direct action of rain.
* The surface of hard rubber becomes porous after exposure in consequence of the sulphur being given off to the atmosphere. - ED.
This condition gives rise to want of insulation in the worst form. Glass and porcelain are more liable to loss of insulation from this cause than any other materials in common use. If the surface of the body is of such nature as will not allow of its being wetted readily, then the objections on account of this deposit of moisture from good conduction of heat are much modified.
This repellent nature or property prevents the moisture or liquid from coating the surface as it would do were the surface capable of being wetted, but compels it to flow over the surface of the insulator disconnectedly in drops, and without forming a continuous conductor for the electricity. Glass, gum and gutta-percha soon lose their repellent powers from modification of the surface structure by atmospheric influences. Oils and varnishes soon change and decay. The only substance fulfilling the conditions necessary to perfect insulation that I have yet tried is paraffine, and this can only be used to render perfect other insulating bodies not possessed of suitable surfaces. This substance, in consequence of its low melting point, requires a modification in the form of the insulator. To insure strength and give greater protection to the insulating material it is better to adopt iron for the body and case of the insulator, securing the line wire in such a manner as to insure perfect insulation by the
 |
 |
 |
 |
 |
 |
interposition of substances so prepared as to fulfill all of the prescribed conditions.
The form of the insulator should be such as to secure a complete horizontal zone from contact with rain or snow. This is most readily done by a system of shedding or overhanging so undercut that water will flow over it in such a manner as to prevent it from being wetted from top to bottom in a continuous path. The form should also be such as to render it easy to remove and replace the insulator when broken by violence or by atmospheric electrical discharges.
The insulators sent to me as samples appear to be fair specimens and are in good condition, and were as follows (the numbers affixed for easy reference during experimentation):
No. 1 — Wade Insulator. — Wooden cover over glass on bracket
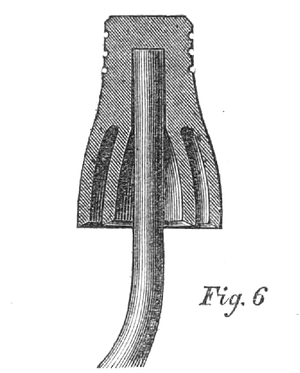
No. 2 — Leffert's Insulator. — Wooden block covering a glass in which the iron hook is secured in molding.
No. 3 — Glass and bracket.
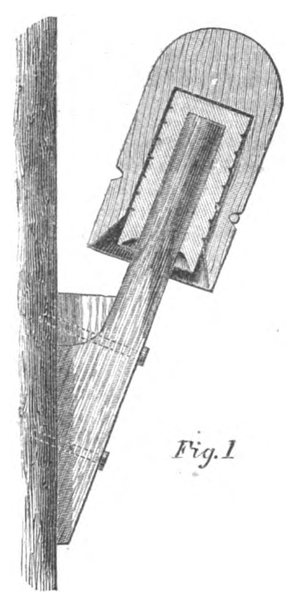
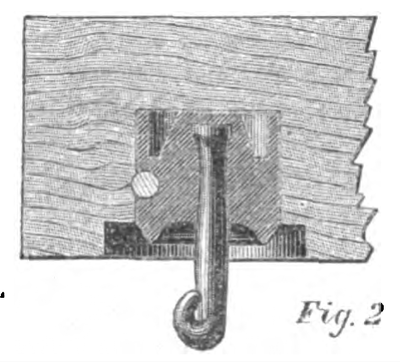
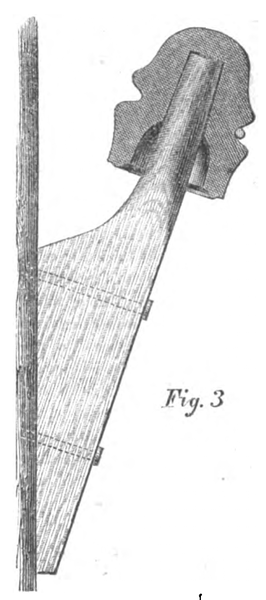
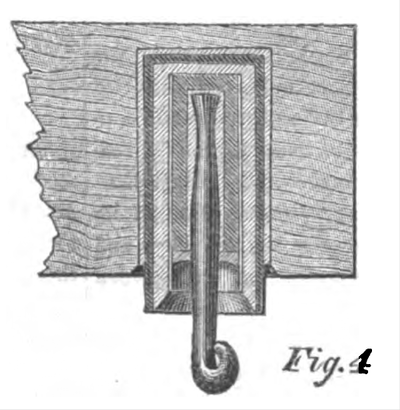
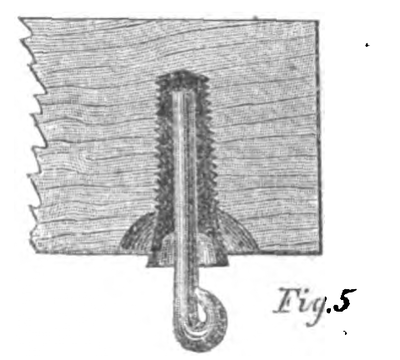
No. 4 — Brook's Insulator. — Iron case, with glass and sulphur and paraffine, with iron hook.
No. 5 — Rubber-covered screw and hook.
No. 6 — English double shed (stoneware).
The trials for leakage were made by placing them in circuit of a battery of eighty Grove cups, around the groove, and connecting the supporting bracket, hook, or block to a ground wire, with a very delicate Thermo multiplier in the circuit.
When dry all of the samples were perfect insulators, but when soaked in water for ten minutes Nos. 1 and 2 gave a leakage too great for measurement with the very delicate instrument employed, whilst Nos. 3, 5, and 6 allowed for the passage of a strong current; No. 4 alone retaining the current.
When exposed to a dry, cold atmosphere below 32° Fahrenheit for twenty-four hours, and then transferred to a warm room and exposed to a jet of steam, so as to produce a great deposit of moisture upon the insulators, Nos. 2, 3, 5 and 6 allowed strong currents to pass; but when a rain shower was included in the change from cold to a warm atmosphere, No. 1 also gave a great leakage, whilst the leakage from Nos. 2, 3, 5, and 6 was much increased; No. 4 again retaining the current.
Repeated trials made upon the whole of the samples, side by side, exposed to a continuous shower of water, gave results as follows (the comparison being made in the consumption of zinc derived from actual trials in the production of equal deflections of the astatic needle by battery power):
[make table]
No. 1 (Wades), .............................. over 35
No. 2 (Lefferts'), ........................... " 30
No. 3 (glass and bracket), ................ " 15
No. 4 (Brooks'), ........................... less than 1
No. 5 (rubber-covered hook), ............... " 28
No. 6 (English), ............................. " 16
From this it would appear that the wooden covering of Nos. 1 and 2 actually took away from the insulating powers of the enclosed glass, which glass was very similar in form to No. 3.
A close examination of Nos. 3, 5, and 6 revealed a surface full of minute pores or cracks capable of absorbing water and retaining it.
The Brooks Insulator alone remained intact during all conditions, and it was assumed for the sake of comparison that the deflection was 1°, although the motion of the needle was too small to be read off the scale; whilst the insertion in the circuit of No. 1 caused a deflection of over 45°. The success of the Brooks Insulator was entirely due to the use of the parrafine, with which the glass and sulphur (in different specimens presented for trial) was completely saturated, filling up all the minute pores and crevices, and rendering the surface of the insulating medium repellent of water.
Trials made with slips of porous wood and porcelain, extremely thin but saturated with parrafine, showed them to be perfect non-conductors of electricity; also slips of the thinnest paper so treated completely prevented the passage of the current from the eighty-cup Grove battery, the poles being pressed together with a single thickness of prepared paper between them.
By proper manipulation with paraffine any of the above insulators may be made temporarily perfect; but to insure permanence it is better to adopt a form of case and block which shall combine as practically as possible the requirements for non-conduction and easy adjustment.
The Brooks insulator, constructed with an iron case with the wire hook secured into it by glass or sulphur, and the whole thoroughly saturated with paraffine and provided with a shedding collar of porous earthenware, likewise saturated with paraffine, seems to me to combine all of the requisites for complete success.
Respectfully, CHARLES M. CRESSON.
