[Trade Journal]
Publication: Electrical Engineering and Telephone Magazine
Chicago, IL, United States
vol. 12, no. 86, p. 179-188, col. 1
Line Insulators.
BY F. A. C. PERRINE, D.Sc.
In the discussion of the pole line we have considered only the mechanical features, our attention having been devoted to the study of the best means for maintaining wires in position where they will be permanent under all atmospheric conditions, and where they will be reasonably free from all interference.
The essential point in the electrical study of this line is a consideration of the best means for cheaply preventing electrical leakage from any wire to the earth or to neighboring wires, and in consequence, this subject has been one to which much ingenuity has been devoted since the earliest days of the construction of overhead lines.
In the first lines erected by Morse, the insulating properties of the wooden poles were thought to be sufficient, but it was soon found that while satisfactory transmission without excessive leakage would take place during dry weather, something more than even a layer of good wire insulation was necessary when the poles and cross-arms became saturated with moisture. At first the want of additional insulation was filled by the use of small blocks of porcelain or earthenware, which could be screwed to the poles, and which supported the wires, laid in grooves or strung through holes provided for this purpose.
In the early days of telegraph working, many miles of wire were supported in this manner both in American and European construction, but it did not take long for telegraph engineers to find that this form of insulator possessed several important defects. As long as the insulators were clean and fresh from the factory they gave perfect satisfaction in dry weather and reasonably good results when it was wet; but as soon as a layer of dust had accumulated over the surface, the slightest amount of moisture would convert this dust into a conducting film, and reduce the insulating properties very seriously. Furthermore, when the wires were strung through holes in the insulators, the difficulty of originally placing the insulators was very great, while a broken insulator could only be replaced by cutting the wire and interrupting the service, while when the wires were simply laid in grooves, they were easily thrown off during storms. Besides, these insulators gave no support when any one pole was overstrained or weakened in any way, since they simply carried the wires, which were not tightly attached to them.
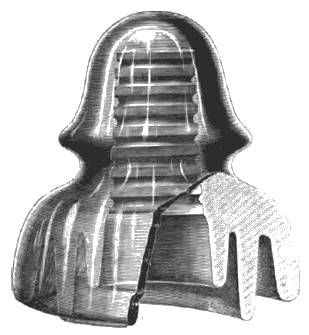
The first efforts toward improvement were of the nature of attempting an increase of surface by making the insulators long, in the shape of a double hollow cone. This materially increased the length of the leakage surface, and especially maintained a reasonably dry interior, except during the hardest storms, but as no method of tying to the insulators had been provided in this form, they were soon abandoned in favor of a simple spool of porcelain held by a screw, with the wire twisted around the groove. This gave additional mechanical support to the line, and facilitated the replacement of broken insulators, but retained the defect of a ready path for leakage over the surface. In consequence, the porcelain spool was soon relegated to indoor services, where it still exists in the form of the porcelain knob, which, as we know, is a satisfactory insulator for indoor construction, where it will remain dry and clean. Before this insulator was abandoned, attempts were made to protect it from moisture by covering each insulator with a wooden or metallic hood of such a shape as to readily shed rain away from the point of connection between the line and the pole, and while this was effectual to prevent a drenching of the insulator during rainstorms, it did not prevent an accumulation of moisture from fog or mist on the dirty surface of the insulator, while it was especially defective, as it prevented a complete washing of the insulator by the drenching rain, which from the very first was found to be one means of improving the insulation resistance of a dirty insulator. At the same time, the complete abandonment of the hood was not attempted until it was found possible to make the hood a part of the insulator itself. In consequence, the next step in improvement consisted of making a porcelain or earthenware hood with two lugs, by which it could be fastened to the pole, while the wire was supported by means of a metallic pin cemented in the interior of the hood. The insulator resembled closely an inverted coffee cup, with the wire supported by a pin cemented on the inside of the cup. This form still exists in the insulators used for supporting trolley wires in all metallic-sheathed insulators and in the so-called "paraffin" insulators, which are often employed where wires are entering telegraph offices. The porcelain lug, by means of which the insulator was fastened to the poles, was found to be defective mechanically, and although this was replaced by a groove and the insulator tied to the pole with a wire, the construction of lines carrying many wires was soon found to require some form of insulator which might be attached to a cross-arm. By reversing the positions of the insular support and the support of the wire, this result was accomplished. The wire was fastened in the groove around the outside of the insulator, while the insulator itself was supported by means of a pin set in the cross-arm, the pin itself being cemented or screwed into the interior of the insulator. This form is the common form of insulator employed at the present time, improvements which have been made consisting mainly in an extension of the surface, along which leakage can take place by a multiplication of watersheds, or, as they are commonly called, "petticoats." One of the latest forms of these petticoat insulators is shown in our illustration (Fig. 1). It is designed for currents having a potential of 5,000 volts or less. It is 4-1/2 inches in diameter, and 3-1/2 inches high, and presents 8-1/2 inches of surface between the wire and the pin. Like the porcelain insulator, this insulator has most of its surface on the bottom side.
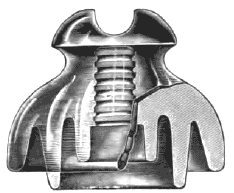
In Fig. 2 another triple petticoat glass insulator is shown, made for potentials up to 20,000 volts.
 |
| Fig. 1. |
 |
| Fig. 2. |
Since the establishment of this design various materials have been employed in construction, and individual designers have changed the depth or location of the groove, and have varied other mechanical details, but it is now considered that all good designs for line insulators to be supported by pins on cross-arms adhere to the following conditions:
The material used must have high specific insulation resistance, and present a surface not readily destroyed and on which no great amount of moisture is condensed from dampness in the atmosphere, while the mechanical strength, both under the influence of strains and vibratory shocks, must be as great as possible. In designing a particular form of insulator, the groove for the wire should be of sufficient depth that it will hold the wire securely in place when the wire is tied, by means of a loop or tie wire. The location of this groove should be such as to transmit the strain through the insulator to the pin, without inducing shearing strains in the body of the insulator. The form above the groove and of the outer petticoat should be such that during a heavy rain the space under the petticoat will be kept as dry as possible, while the external surface of the insulator is being thoroughly washed. The space under the petticoat should not be so narrow that a ready circulation of the air be hindered, and the insulator caused to dry slowly after being dampened by mist. Finally, as little shelter as possible should be provided for insects that seek dark places in which to lay their eggs and form their cocoons. These conditions are largely of a mechanical nature, and it is obvious that the manner of their fulfillment depends largely upon the character of the material used, since each insulating material presents certain mechanical advantages and difficulties.
The principal materials that have been used in the construction of line insulators are glass, porcelain, pottery, hard rubber, "compressed mica," and lava. Of these, rubber is only suitable when it is possible to protect it from atmospheric influences, since all forms of rubber and ebonite are found to decompose on the surface and produce an external conducting film of sulphuric acid, when subjected to the influence of the sunlight, and consequently this material can only be properly employed in those insulators which have an external sheeting of iron or other metal, although when so employed, rubber has the advantage of withstanding easily severe vibratory strains that might be fatal to the more brittle glass or porcelain. Furthermore, it is easier to make the connection secure between the rubber and the iron sheeting than is possible with any of the harder insulating materials.
Lava of certain especial qualities, carefully selected and tested, seems to produce good insulators, which are exceedingly tough and strong, but its use has not been very extended on account of the fact that the lava insulator has to be machine turned from a large block of the material, and although lava works with comparative ease, the expense of this machine work is so great that its advantages are not generally considered to be commensurate with the increased cost of turned insulators.
The term "compressed mica" is a trade name for a composition of ground mica and shellac, molded under the influence of heat and pressure, the outside surface of shellac being smoothly glazed. This material has been very effectively used where great shocks are to be sustained, as for the insulators supporting trolley wires, but as the smooth shellaced surface of this material has no great weathering properties, it is not thoroughly suitable for line construction unless covered by a metallic sheeting, and even in such service its insulating properties, after repeated wettings by the rain and mists, are not sufficient for it to be considered as a satisfactory material for insulator construction, except in resisting low potentials on lines subjected to great vibratory strains, where the slight leakage over the surface of the insulator is more than counterbalanced by its mechanical strength.
The material called "pottery," which is made from common clay, or hydrated silicate of alumina, from which the water of crystallization has been driven away by baking, is quite porous and only considered to be a good insulator when kept dry by means of a heavy external glaze. On this account the material is not in favor in this country, although many telegraph lines abroad are supported on pottery insulators, where it is favored on account of its great cheapness, as well as on account of the readiness with which colored glaze may be applied, different colors being preferred by different telegraph superintendents. But although, as has been said, the insulating properties are good when the glaze is intact, our engineers consider that the glaze is too readily ground off by the swaying of the wire or cracked away by carelessness in construction, for this material to be valued in the construction of insulators.
In place of pottery, glass is here commonly used, being preferred on account of its cheapness, its strength, its smooth surface and its transparency. The particular glass usually employed is a cheap form of soda glass made of reasonably pure sand, but without attempt to remove the last traces of metallic impurities. In consequence of the presence of a small amount of iron oxide, these insulators have a green color, although not of such a depth as to render them opaque. This glass, while more deeply colored than lead glass, is much cheaper and at the same time stronger, while its surface is less hygroscopic and more enduring, both under the influence of the solvent action of moisture and the grinding action of the line wires. In this country it is considered that the transparency of glass presents a decided advantage, as in consequence of this property there are no dark recesses within the insulator, and there are, therefore, no places especially attractive to insects, whose nests and cocoons cause serious inconvenience and loss of insulation where opaque insulators are employed. In some sections of the country this property is so important that efforts are made to obtain a glass nearly white, while all over the United States the presence of vast numbers of insects is of sufficient importance to render this property of glass valuable. The principal objections to the use of glass are that the material itself is quite brittle and the surface condenses and retains moisture more readily than that of other insulators.
By properly proportioning the size of the insulator to the strains likely to be endured along the line, the influence of the fragility is reduced to a minimum, although this is always a disadvantage where the lines are likely to be interfered with by falling wires, trees, or by malicious breaking of the insulators by stones or shot. On long lines the presence of malicious influence is not of great importance, and indeed this principle need only be considered where wires are run through the suburbs of a large city, although it would be desirable to obtain for good insulation a material less hygroscopic. It is common to consider that the hygroscopic character of the surface is of less importance in this country than the presence of insects, and until this problem shall be solved in some other manner, glass is likely to continue to be the standard material for the construction of small insulators, which cannot be made of an opaque material without dark recesses.
 |
| Fig. 3. |
 |
| Fig. 4. |
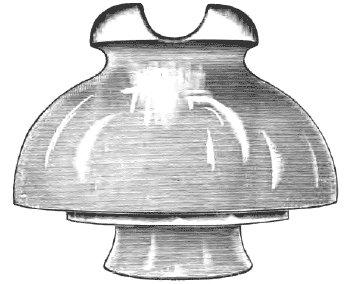
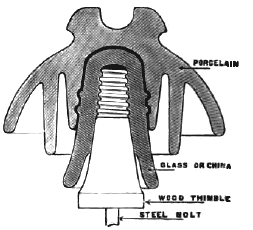
For heavy mechanical strains and high potentials, some of the principal objections to the use of glass are obviated in insulators made of porcelain. In the development and perfection of these porcelain insulators for high pressures, probably the greatest advance is shown in the Locke types, which are now made for potentials up to 100,000 volts. Fig. 3 represents a Locke two part insulator, Fig. 4 showing a sectional view of the same, which has been designed to carry potentials up to 50,000 volts, and which is made in several sizes according to the different currents to be carried. It is made with an outer shell of china ware on account of its high insulating surface, mechanical, as well as electrical strength, and has a center insulator of glass to further insulate it and to prevent puncture by the high potential current. It is also made of two or more shells of china ware, the advantage of this over one solid insulator is that the shells being made separately one-half inch thick, each allows a greater uniformity in making and more thorough vitrifaction, than could be obtained otherwise in one solid insulator one inch thick. Besides this Mr. Locke secures four thicknesses of glazing from the two shells, which further increases its insulating qualities. One thickness of shell being capable of standing, say one volt, the two together will stand double that amount. By repeated practical tests it has been found that a potential of 100,000 volts will not puncture the insulator; hence it will carry with safety and economy any voltage that is practicable for use in commercial application of electric power. Porcelain is a material which consists essentially of a hard body, made by the dehydration of hydrated silicate of alumina, in which the pores are filled by a suitable glass, producing a solid nonporous substance of considerable strength and toughness. This material differs from pottery in being more dense, whiter and less fusible, but particularly in being translucent. Indeed, many consider that the quality of translucency is the only one by means of which porcelain may be distinguished from pottery. Pottery, as we have said, is a ceramic ware, molded from paste of impure hydrated silica, containing certain amounts of free silica, lime and iron, together with a frequent admixture of organic matter. After this is baked to drive off the water of hydration, the product is opaque and invariably porous, on account of the removal of the volatile ingredients contained in the wet clay from which the ware is molded. Porcelain, on the other hand, consists partly of an almost pure silicate of alumina, but slightly hydrated, inclosed within a matrix of a hard silicate glass, the glass serving to fill up the porosities in the dehydrated silicate of alumina, and to make the material thoroughly nonabsorbent. Porcelain as used in the arts, however, is not a constant material, but varies from the variety called "hard-paste" (originally invented in China) to an altogether different material discovered by the French, and called "soft-paste" porcelain. The true, or hard-paste Chinese porcelain, is composed of a mixture of kaolin with a natural silicious glass found in China and called Petunese, while the soft-paste consists simply of a mixture of the kaolinic clay with an artificial glass composed of niter, soda, gypsum and salt, the proportion of kaolin to the glass being much less than in the natural Chinese product, and in consequence the resultant product partakes more of the character of glass in brittleness, although it is as free from porosity as is the true porcelain. Between these two are found the mixed or "bastard" porcelains, which are uncertain in character, but are all composed of kaolin, inclosed within a more or less fusible glass, and consequently varying in properties.
Porcelain insulators are rarely "thrown" on the potter's wheel from masses of clay, as was the ancient custom in ceramic manufacture, but are made by pressing the clay into a matrix by means of a die. For this purpose the clay, which has been thoroughly ground and mixed in water, is dried to the condition of a damp powder, which is then filled into the matrix and pressed into the form of the finished article by means of a die. In this method of working, a minimum amount of water may be used, and in consequence the proportion of fusible material may be decreased with the result that the finished article is made more dense and tough, but at the same time it is impossible for the moisture to be entirely eliminated from the clay until it is subjected to the heat of a potter's kiln. That this process removes a very considerable amount of water from the mass, is shown from the fact that the shrinkage in baking amounts to as much as the shrinkage in the cooling of cast iron, namely, a shrinkage of one-eighth in the linear dimensions. The spaces left by this water are filled by the fusion of the glass with which the kaolin is mixed. When this mass of clay is baked to drive off the water of hydration, and the biscuit thus produced is dipped into a thin paste of glaze and again fired until all the fusible material is thoroughly vitrified, the resultant insulator presents a smooth- glazed surface and a body without porosity. When so manufactured, there is no doubt that an insulator is made which is tougher and stronger than any that may be made from glass entirely. Besides, the surface of the glaze, although this is indeed a glass, is found to condense water from the atmosphere much less readily than the surface of solid glass. The proper porcelain for insulation, therefore, is that in which there is only such an amount of glass present as is necessary to fill up the porosity of the dehydrated silicate of alumina, since when this proportion is attained, the greatest strength consistent with non- porosity is reached. Should the amount of glass be increased beyond this point, or should a more readily fusible glass be used, the porcelain will become brittle, although it will still remain nonporous, but nonporous just as glass is nonporous, and, hence, without the advantage over common glass supposed to be possessed by a hard-paste porcelain. If the amount of glass present is only so much as will fill up the pores left by the escaping water as the silicate of alumina is dehydrated, we can readily see that such porcelain has no property by means of which wide cracks in the molded clay can be filled, since at no temperature available within the pottery kiln will the mass fuse and run. We may indeed say that the glass is drawn into itself by the porous silicate of alumina by capillary attraction, and when spaces are present which are not capillary, these spaces cannot be filled. The solidity of the finished article in hard-paste porcelain manufacture depends, therefore, upon the solidity of the molded clay.
Hard-paste porcelain is made of materials expensive in themselves, difficult to mold and fusible only at a very great heat, therefore, the temptation to increase the amount of glass in the material and to use glass fusible at a comparatively low temperature is very great, although the material resultant from such change is found to possess the valuable properties of porcelain in a very seriously diminished degree. In consequence, many of the bad results found in insulators are due to a fragility and excessively hygroscopic character caused by a degradation of the material. This degradation is exceedingly difficult to detect, since the highest experts on porcelain wares are often at a loss to determine whether the body of a finished article is hard or soft paste porcelain, without determining its crushing strength, and, of course, a mechanical test is very hard to apply to such an irregular body as a line insulator. Even where the best materials are invariably used, certain forms of insulators cannot be molded in the matrix and die, for the reason that the clay when pressed is not a liquid body, and in consequence pressure applied all in one direction is likely to produce cleavage lines in the insulator, which are especially apt to occur where irregularities of pressure are sustained, due to a variation in the thickness of the parts of the insulator. This difference in thickness of the various parts of the insulator is also a source of danger during baking, an effect of the great shrinkage already mentioned.
Another difficulty in the manufacture of porcelain insulators is found in the choice of a proper glaze. The glaze must not only present a smooth surface, but also be of nearly the same coefficiency of expansion as the body of the insulator, in order that minute cracks may not occur when the finished articles are taken from the kiln and exposed to the action of the atmosphere. It is true that the glaze does not prevent leakage through the body of the insulator itself, but we depend upon it for preventing the retention of moisture upon the surface, and the non-hygroscopic character of porcelain depends, therefore, upon the character of the glazed surface, and also upon its mechanical perfection. To obtain a glaze which will not be roughened by the action of weathering and will not be worn away by abrasion from the line wire is, indeed, an impossibility; at the same time, care should be taken that these defects are not accentuated by minute cracks within the glaze itself. The proper porcelain insulator is, therefore, one which is made of hard-paste porcelain of great mechanical strength, formed so that internal cracks are not produced during manufacture, and which has been finally glazed with a thin coating of a smooth, hard glass.
The difficulties encountered in the manufacture of a porcelain insulator, due to the varying thicknesses of the parts, are overcome in certain forms by manufacturing the insulator in more than one part, the different parts being fastened together by means of cement or by means of a thick coating of glaze. This method of manufacture was attempted in the earliest history of the use of insulators, and while this may overcome certain difficulties in the manufacture, there seems to be no essential difference between the resultant multi-part insulator and that which has been properly made from one piece. In either case, the insulators are found to withstand high voltages and to properly sustain the line whenever the leakage surface and the thickness of the insulator is made sufficient.
