[Trade Journal]
Publication: Transactions of the American Ceramic Society
Columbus, OH, United States
vol. 11, p. 185-201
Transactions of the American Chemical Society
CRYSTALLINE STRUCTURE IN PORCELAINS.
BY
ARTHUR S. WATTS, Victor, N. Y.
Concerning the history of porcelain investigations, I find by the writings of Plenske that Ehrenherg in 1836 observed that the kaolin and flux lay near one another in a milky appearing mass the flux forming the transparent portion and the opaque kaolin in the form of globules strung one after another, while crossing in all directions, woven one into another, appear tiny straight sticks. In 1847, Oschatz and "Wachter offered data tending to prove that porcelain consists of a glassy matrix filled with innumerable fine crystals, which so reflect and refract the light as to cause the body to appear opaque. Behrens, in 1873, confirmed these investigations, but held that the refraction of light is largely due to the presence of quartz splinters, which he found exists in great quantities, especially in Berlin porcelains. In 1876, Truax came forward with the claim that porcelain is a mechanical mixture of uncombined quartz and kaolin particles, the intervening spaces being filled by the molten but not chemically combined feldspar.
Hussaks found crystalline structure in high fire porcelain and stated in 1889 that he was impressed with their similarity to Sillimanite. Mellor also found crystalline structure in Chinese and Berlin porcelains, in a fire brick and in a slag from Vesuvius. Vernadsky, in 1890, came forward with the observation that in different porcelains there exists apparently prismatic crystals, optically positive, disappearing in polarized light parallel to the prism faces and insoluble in cold HF. Through isolation by means of HF, H2SO4, and neutralization through (NH4)2 CO3, decantation and washing, Vernadsky found in Sevres porcelain 35% crystallite, which had the composition 29.7 SiO2 and 70.3% Al2O3. This corresponds to the formula 11 Al2O3 S SiO2. Vernadsky held, however, that some powdered alumina was also present and that the needle crystals were really Sillimanite, i. e., Al2O3 SiO2.
Vernadsky later obtained isolated crystals, which by analysis showed a composition of 37.31% SiO2 and 63.65 % Al2O3, which gives practically a formula of Al2O3 SiO2. He also obtained Sillimanite crystals from andalusite, cyanite, topaz, and other minerals at 1320° to 1380° heat, and excellent crystalline structure was obtained by heating a mixture of pulverized alumina and silicic acid, in the proportions 1 mol. Al2O3, to 2 SiO2. Plenske also found in fluxed Zettlitz kaolin a formation of crystallite in the amorphous mass.
From the great resemblance of the crystal needle obtained in the molten minerals to the crystallite obtained in burned clay, alumina-silicic acid mixture and in the different porcelains, Vernadsky came to the conclusion that "All clayware is through sufficiently severe burning filled with Sillimanite crystals."
Glasenapp, from his study of the microstructure of clayware and kaolin and apparently without any knowledge of the observations of Vernadsky and others, makes this statement of his conclusions: "All clay is, in sufficiently high temperature under dissociation of the clay substance, crystalline." It exists an a glassy matrix and a crystalline portion, and since the fusibility of the aluminum silicate decreases with the silicic acid content, Glasenapp thinks that the crystalline portion is rich in alumina and the amorphous portion is rich in silica.
Just how the process of crystallization and re-construction occurs, we may only infer from the varied opinions offered. Plenske rinds that where neither the pure kaolin nor the pure feldspar contain crystallite, the formation of Sillinianite may occur in the presence of two materials heated together, and he can only explain this through the chemical influence of the molten feldspar on the amorphous Al2O3 2Si02. He finds also that an addition of 1—2% basic substance as alkali or lime promoted the decomposition of the Al2O3 2 SiO2, and hence encourages the crystalline formation. The addition of a small amount of magnesia gives much more beautiful crystals. Plenske also notes the importance of the rate of cooling on the resultant product. The action of the burning process on the constituent ingredients is well explained by Behrens, who from microscopic investigation concluded that the clay substance, on account of its small grains and amorphous condition, is more dissolved by the molten feldspar than is the quartz.
By the firing of a porcelain containing quartz most of the quartz particles are externally dissolved and covered with a coating of silicate preventing any further attack, and resulting in the matrix never becoming sufficiently fluid for a diffusion of the different silicates.
Plenske finds, as a result of investigations carried on, that about the only certainty established is that the kaolin is dissolved by the molten feldspar. He further notes that the crystallization in many porcelains is distributed throughout the entire mass, while in others it is only noted in groups or isolated appearances. It appears, therefore, no positive statement can be based on observations of the porcelain structure.
Zoellner in his investigations went more into detail, so far as the action from different clays were concerned. He tested seven specimens of kaolin and clay and from the resultant masses determined the percentage of Sillinianite present. I give below a table showing this data:
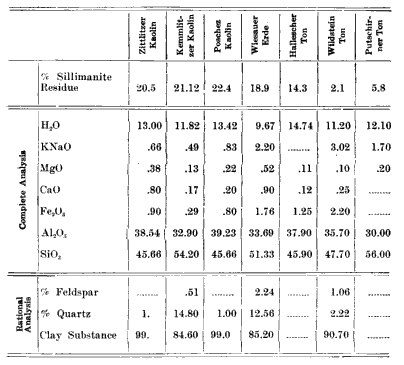 |
As a result of his investigation, he says: "One will not go wrong when one asserts that the amorphous product of the neutral mass, is converted into a portion rich in silicic acid and a crystalline portion, which is a rich aluminum silicate."
He quotes Hecht as explaining the different limits of soft and hard porcelains from the existence of an undissolved quartz, but expresses a doubt if the decomposition of the quartz alone is sufficient to bestow on the burned mixture the characteristics of porcelain. Also Geo. Vogt, Director of the National Manufacturers of Sevres, observes in this connection that one and the same mass burned at different temperatures displays different characteristics. Zoellner finds that the transparency and the fracture are greatly influenced by sillimanite structure. He finds that the specimens without sillimanite structure exhibit a faint earthy yellow translucency and granular fracture, while those with sillimanite structure display highly uniform bluish-white translucency and a smooth fracture. In his investigations he accumulates data which leads him to the following conclusions:
Feldspar is able, in the heat of the porcelain kiln, to dissolve 3.5% alumina, 14% clay substance, and 60 to 70% quartz. This ratio does not alter where more than two components go into reaction.
Microscopic investigation indicates that at Seger cone 15 — 16, feldspar porcelain consists of a glassy matrix, filled with plentiful needle crystals, violently corroded quartz and many gas bubbles.
The minute structure of low fired porcelain, i. e. to cone 12 — exhibits melted feldspar, corroded quartz, and an amorphous silicate, probably anhydrous clay substance, all lying close together.
The analysis of the crystalline portions from two different porcelains, and a high-fired Seger porcelain, give a ratio of Al2O.i to SiO2 as 1:1. The crystals are, undoubtedly Sillimanite.
Concerning the formation of Sillimanite, Zoellner concludes by stating that in the Seger kiln it develops at Seger cone 13 (about 1400°). In the porcelain kiln, however, the development may occur at 1350°—1370° on account of the longer time of burning. He says, however, that they do not form below 1350°. In the porcelain kiln all of the Sillimanite is formed from the kaolin.
The origin of Sillimanite depends not on a devitrification phenomena, but from a molecular alteration of the day substance. The splitting of the clay substance into an amorphous silicate, rich in silicic acid, and a crystalline silicate, rich in alumina, is hastened by the working of the flux.
Zoellner reaches the following industrially important conclusions:
He confirms the established impression that it is necessary to advance to higher temperatures to obtain a good product.
The optical or chemical investigation gives a method by which to differentiate between high fired and low fired porcelains, viz: such with Sillimanite being hard porcelains, and such without Sillimanite being soft porcelains. All the above investigation merely created in me a desire for personal observation of some of these interesting phenomena. I prepared slides from such porcelains as I was personally acquainted with, and although my ability to interpret the observations must necessarily be very meager as compared with the work of others along this line, I offer to you my observations and conclusions in the hope that, some light may be thrown on the practical side of this investigation.
From my observations, I conclude that porcelain consists of a matrix of fused spar and possibly other fluxes, with clay and flint suspended and more or less dissolved in it. If the heat has gone high enough, the degree of fusion may have reached the stage where we have a viscous glass in which is dissolved practically everything that enters into the compound; or if the other extreme is reached, we have nothing more than a conglomerate bonded together by a glassy substance made up of semi-molten feldspar, aided by any other fluxes present, and perhaps carrying small quantities of clay and flint in solution.
We know that the translucency is largely dependent on the alkalies, that the substitution of alkaline earths tends to produce stoniness, and also that the ratio of flux to clay to flint regulates the warping tendencies of the body produced.
What I desire to bring to your attention is the process by which these changes occur, and the extent to which the dissolving process is influenced by the addition of alkaline earths or the substitution of one alkali for another.
We cannot, at this time, cover the entire field, but I shall attempt to show you by the aid of the microscope and polarized light, what the relative action of a few different fluxes may be under given heat conditions.
For this study we will first look at porcelain marked "O," (Vol. X, Trans. A. C. S., page 266) which has the composition:
Feldspar ....... 21.35
English China Clay ... 43.92
English Ball Clay .... 8.00
Flint ......... 26.73
We see here, standing out in bold lines, coarse granular matter, but on closer study we find that there are a great number of clusters of tiny needle crystals submerged in a liquid matrix, and the whole more or less obscured by a great number of tiny spherical particles through which light passes with difficulty. We also note numerous larger spherical bodies suspended in glassy matrix.
What may we assume these various materials to be?
We can safely assume that the finely ground feldspar has nearly, if not quite, reached the liquid state, since cone 12 was reached by all of the specimens here shown unless otherwise noted. The flint is the only other crystalline material introduced, hence I think we may safely assume that the majority of these angular coarse grains are particles of undissolved flint. We cannot hope to find the flint in true crystalline form, owing to the, grinding process through which it has passed, but the strongest argument for my contention that these are particles of flint, lies in the fact that they display sharp angles and smooth faces, which we cannot expect to find either in the fused spar or the clay substance.
The tiny semi-opaque spheres, which cloud the matrix, are undoubtedly clay-substance, and the large spherical particles above referred to are undoubtedly gas bubbles, coated with the opaque clay substance. These gas bubbles have almost clear surfaces where the fusion has progressed to a sufficient degree, but with feldspar alone as a flux and in the proportions of "O" porcelain, the gas bubbles are coated so heavily with undissolved clay substance that they are practically opaque.
We will now add to this "O" porcelain, two per cent of CaCO3 as whiting by which we have "K" porcelain (Trans. A. C. S., Vol. X., page 267). Note the change in the appearance of the flint or quartz grains. The edges are no longer distinct, showing that the matrix has begun to attack and dissolve some of even these large particles of silica. Also note the lack of contrast between the matrix of fused spar and the clay substance. The spar matrix in porcelain "O" was nearly obscured by the substance while in porcelain "K" the addition of only two per cent of CaCO3 has enabled the matrix to dissolve much of this clay substance. Also note the isolated clusters of needle-crystals where the fused spar matrix exists in large amounts.
We will now look at porcelain "L" (Trans. A. C. S., Vol. X, page 267), which contains two per cent of CaCO3 in the form of pulverized marble. This should be similar to "K" porcelain, except that it will show any difference which may arise from the MgCO3, of which the marble contains 17%. This we find to be true, except that the matrix is not quite so clear as is that of porcelain "K." This phenomenon may have some connection with the note in Vol. X. to the effect that "L" porcelain seemed whiter than "K" or "M" porcelains. This cloudiness is due entirely to the large amount of beautiful crystallite present, and which is very much more uniformly distributed than in "O" or "K" bodies. Note also that the masses of fused spar matrix are less distinctly outlined than in "K" body, indicating more fusion.
This notably increased number of needle crystals and the increase in size, and distinctness of same confirms the observation noted by Plenske that "an addition of magnesia gives more beautiful crystals".
We will next study "M" porcelain (Vol. X, page 267) in which the calcium is introduced as CaSO4 or plaster of Paris. This porcelain is very similar to "O" body, indicating that the flux in this form is less active than when introduced either as whiting or marble dust.
Our next sample will be porcelain "P", which by Vol. X, Trans. A. O. S., page 98, you will find contains:
Genuine Cornwall Stone ---------------- 43.69%
Whiting -------------------------------------- 0.10%
English China Clay ---------------------- 34.00%
English Ball Clay -------------------------- 8.00%
Rock Flint --------------------------------- 14.17%
99.96%
This body is of the same formula as "K1', "L" and "M" porcelains, but the introduction of Cornwall stone in place of feldspar has changed the percentage composition.
Note in this body we find a large number of patches of material similar to the matrix of "K", but which are fused almost to a degree equal to that displayed in "L" body. The crystal needles in "P" body are very large and distinct, but not so numerous as in "L" body. The line of contact between the flint particles and the fused matrix is clear and liquid-like. This is a peculiarity of the Cornwall stone, which we do not find in any feldspar porcelains, unless the feldspar has been previously fused.
We will compare the body just inspected with a body exactly duplicate, both in formula and in percentage composition, but in which the Cornwall stone is replaced by an artificial mixture of spar, clay, flint, magnesium oxide and fluorspar fused and ground. This body is known to you as porcelain "Q." (Trans. A. C. S., Vol. X, page 99.)
Porcelain "Q" seems very similar to "L'' porcelain in formation, the matrix being almost entirely of clear fused glass which is exceptionally free from color or cloudiness. The crystals in this specimen are exceptionally large and distinct, and seem to form a fringe projecting into the masses of fused matrix. Only when the mass of fused matrix is very small is its entire mass filled with crystal structure. I would note here, however, that the extreme clearness of the fused matrix in this body undoubtedly adds much to its ability to display its crystal structure, and evidently indicates almost a total solution of the clay substance. I also note the presence of an exceptional number of gas bubbles in this specimen.
We will now look at a specimen which I will call porcelain "S" and which is exactly identical with porcelain "K" except that it contains feldspar, which has been previously fused alone and reground. This "S" porcelain is fired at cone 10, whereas all previous specimens have been fired at cone 12. The crystals in this porcelain are small in number and only of moderate size and the flint particles appear to have been very perceptibly acted on by the fluxing action of the matrix. The matrix is similar to "O" in structure, indicating that at cone 10 this dissolving action of the matrix noted in "K" does not occur. We note, however, the same clusters of distinct bubbles that we noted in "Q" where the flux consisted partly of fused and reground spar.
We will next look at porcelain "U," cone 10 fire, which consists of the same materials as "K," except that the feldspar used is a softer spar. This body is explained in another paper read at this year's meeting, and is very similar to "O" body, but the matrix is clearer and the quartz, grains are more attacked by the matrix than in "O." Note that the quartz grains are not surrounded by the clear area as in "Q'' body, but that a cluster of semi-clear globules, as though the presence of the matrix at this temperature tended to only partly dissolve the opaque clay substance. The clay substance in the remainder of the matrix seems to have lost some of its opacity, although its cloudiness is still distinct.
We now come to the soda spar body, referred to in another paper read at this year's meeting. This is known as porcelain "V," cone 10 fire, and is particularly interesting because it shows more effects of dissolving action than do any other of the samples shown. The clay substance is notably attacked by the matrix, being apparently completely dissolved by it. The quartz grains are very rounded, indicating partial solution. The most interesting point brought out by a study of this porcelain is that we find in it, at cone 10 firing, a very even distribution of large needle-like crystals.
Another peculiarity of this specimen is that there are bubbles or holes appearing in the body of the semi-dissolved quartz grains, indicating that the flint particles are beginning to actually dissolve, and the opacity of these flint particles indicates further the extent to which they have been acted on.
In order to study the progress of solution and crystallization with rise in temperature, I prepared a porcelain of the following composition and called it
"W" PORCELAIN.
Feldspar --------------------------- 33%
Flint --------------------------------- 17%
American China Clay ----------- 25%
American Ball Clay ------------- 25%
---------------------------------------100%
This body was prepared in one mass and then divided into three portions which I had tried in a normal atmosphere at cones 9, 11, and 13.
NOTE — I have used only American Clays in this porcelain in order to compare it with the English Clays used in the other porcelains.
It contains no calcium and should show us the progress of vitrification and solutions and at the same time indicate at what temperature the Al2O2, SiO2 crystal forms. The results of these three firings are all that could be hoped for in the matter of progressing solubility.
"W" Body. At cone 9 we have a more opaque cloud of clay substance than in "O" porcelain, while the exposed quartz grains are much sharper in outline, and with no appearance of corrosion. The clay substance globules in this body are practically opaque and so cloud the feldspar matrix that the tiny needle-crystals present are only dimly outlined. Needle crystals are, however, very evenly distributed, altho very small in size.
"W" Body. At cone 11 we note some improvement, the porcelain now being practically the same as porcelain "O" at cone 1± Isolated patches of distinct needle crystals are noted, altho the cloudiness of the matrix still prevents the smaller crystals from appearing distinct. At cone 13 we note a very pronounced change. The quartz grains appear clear and slightly corroded or dissolved on the edges. The feldspar matrix, however, is notably clearer and the bubbles suspended in it are almost absolutely free from cloudiness. I note in this body the larger clusters of clear bubbles similar to those noted in "Q" body. The feldspar matrix is very evenly filled with crystal needles of good size and distinct outline. The larger particles of feldspar, which in the two preceding specimens retained their general outline, are lost in this specimen, and their presence is only indicated by clusters of fine Sillimanite crystals.
I will now show you for comparison, a vitrified body fired at cone 10 and composed of flint and clay substance, and in which the feldspar is replaced by a fusible iron-bearing clay. Note how clear this matrix appears and also the presence of tiny crystal needles, which are evenly distributed throughout the entire mass. Also note how distinct the fine globules appear, indicating that this form of flux does not possess any great power as a solvent of clay substance. This is in direct variance with all regular porcelains in which we note that as the matrix becomes transparent, the clay substance disappears.
I also want to show you a specimen of bone-china. This was cut from a first class specimen showing fine surface and translucency, and no evidence of warping. Note the great amount of opaque material and the absence of anything that might be classed as crystalline. The calcium phosphate apparently does not exist in a crystalline form in this specimen, nor is there any apparent crystallization as a result of vitrification in this specimen.
SUMMARY.
As a result of this line of study, I offer you the following as my interpretation of the development, pyro-chemically, of porcelain:
Of all the ingredients entering into the composition of the porcelain, the feldspar is the first to act. If it is a hard spar, cone 9 may be reached before it does more than bond together the other ingredients into a conglomerate mass. As the temperature rises, the feldspar becomes more active, dissolving first the clay substance and later the Hint particles until at cone 10 the porcelain begins to show evidence of Sillimanite crystallization. At cone 13 a hard spar becomes completely fused, and is completely fluid permitting such complete crystallization as conditions and other ingredients will permit.
The substitution of a soft potash spar for a hard potash spar is a point worth noting. I find that a difference of two cones in the maturing temperature may be effected, and an equal amount of dissolving action obtained. A study of the relative analyses of the spars would lead one to suspect such a difference. This inclines me to the belief that the virtue of very hard spar lies largely in the fact that a longer, higher fire gives more uniformity of heat distribution. With equal care at lower temperature, a soft spar should give equal results and save considerable in fuel and kiln maintenance. The addition of some other flux, even in small quantity, causes the matrix to become much more active as a solvent. Whiting and pulverized marble are more powerful in this respect than an equal equivalent of plaster of Paris, and they give to the matrix a dissolving power in excess of that obtained by an equal amount of flux in the form of feldspar.
The substitution of a soda spar for potash works a greater change than is possible in any other way. Besides causing the remarkable change in sounding quality, the soda spar gives to the matrix a dissolving power that is remarkable. As evidence of this I call your attention again to the splendid crystal formation which is noted in this specimen. No cone 12 specimen observed, has reached this stage, hence I assume the soda spar body readied a higher stage of solution at cone 10 than the other specimens readied at cone 12.
Regarding the Cornwall stone, the only note I make is that it acts more like a fritt than a spar, although its fluxing action is apparently not equal to that of a fritt of the same composition. In fact, the activity of this fritt in dissolving the flint particles is remarkable as compared to an unfritted flux. The advantage of fritting the spar and lime together, over that obtained by fritting the spar alone, is strikingly displayed in this study. A peculiarity of all bodies containing fritted material is, I note, that they display dusters of segregated bubbles which we only find elsewhere in the soda spar porcelain and in the porcelain containing 33% of feldspar and fired at cone 13.
This segregation of bubbles suggests to me that the matrix is ordinarily too viscous to permit of the gas passing off freely, so that the bubbles expand where they are given off. When fritted flux or soda spar is used, the matrix is sufficiently fluid to permit of the bubbles passing off more freely and the segregation occurs as noted.
In closing, I desire to add a note concerning the action of fluxes on the different clays. From a study of mixtures of various clays with spar in the proportion of 70% clay and 30% spar, and fired at cone 12 in a 40-hour burn, I find that the ball flays display notably less solubility in the fused feldspar than do the china clays, altho the ball clay porcelain displays by far the finer texture. I also note far greater crystalline development in the China clay than in the ball clay porcelains. This same phenomena has been noted by Zoellner in his table of kaolins and clays tested, in which you will note that the Wildstein Ton and the Putschirn Ton develop the least Sillimanite structure, and at the same time possess the greatest plasticity. This would lead one to infer that the ball clay exists in too large grains for ready dissolution or that it possesses some form of structure or some quality which retards the fluxing action of the molten feldspar.
DISCUSSION.
Mr. Watts: I want to say, on concluding this paper, that I desire to acknowledge, through our Transactions, my indebtedness to the Bausch & Lomb) people for their great generosity in furnishing the apparatus we are using; also to Mr. Fred Locke, who has given me repeated opportunities to use his high power microscope for my study of porcelain. I doubt if very much could have been accomplished were it not for his kindness in allowing me to use it. But little could 1me been done without the polarized light machine, although we might have obtained something of the kind, but it would have required a very large amount of detail work on each specimen.
Prof. Orton: I think this is one of the occasions where the member who stays at home and says he can get it all in the Transactions, gets fooled.
I move that the American Ceramic Society tender Bausch & Lomb a vote of thanks for equipping us with this magnificent piece of machinery, without which Mr. Watts could not have brought the slides illustrating this paper; also to Mr. Locke for his kindness in loaning Mr. Watts his microscope.
The motion was seconded by several and then put and carried unanimously.
On motion a vote of thanks was also extended to Mr. Watts for the work he had done.
