[Trade Journal]
Publication: Western Electrician
Chicago, IL, United States
vol. 3, no. 16, p. 206-207, col. 1-3
Bell Hangers' Hand-Book.
BY F. B. BADT.
PART II
TWO-FLUID BATTERIES — (Continued).
CHAPTER XV.
The Leclanche Battery.
In this cell the exciting liquid is a solution of sal-ammoniac. In this the zinc dissolves, while ammonia gas and hydrogen are liberated at the carbon pole.
To prevent polarization in the disque form, Fig, 10, the carbon plate is packed inside a porous cell with fragments of carbon and powdered binoxide of manganese, which slowly yields oxygen, and destroys the hydrogen bubbles.
 |
| Fig. 10. — Leclanche Disque Battery. |
In the prism form, Fig. 11, the binoxide of manganese is applied in plaques or prisms, thus avoiding the necessity of using a porous cell.
The Leclanche cell will give a continuous current only for a short time, the power falling off, owing to the accumulation of hydrogen bubbles; if the circuit is left open for a time the cell recovers itself, the binoxide gradually destroying the polarization.
The cell is in other respects perfectly constant, very clean, and as it does not require renewing for months or years, it is well adapted for working electric bells, annunciators, burglar alarms, and for domestic purposes. These batteries are set up in the following manner:
 |
| Fig. 11. — Leclanche Prism Battery. |
The Disque form. — Put six ounces of sal-ammoniac into the glass jar, fill one-third full of water, and stir. Put in the porous cell and fill with water to the neck of the jar, pouring a little water into the hole in the porous cup. Put in the zinc and connect the battery.
The inside of the rim of the jar is paraffined, and should be kept greased to keep the salts from creeping.
The battery should be kept in a dry place of medium temperature. It requires very little attention; water should be poured in occasionally to supply the loss by evaporation. In case the solution becomes milky, and the battery fails to work, the solution, should be thrown out and fresh sal-ammoniac and water put in. If this does no good, soak the porous cell in warm water. If it still fails, new porous cells must be used.
The Prism Form. — In this cell the porous cup is dispensed with, and in its place is substituted a pair of compressed prisms or plaques, which are simply attached to the carbons by means of two rubber bands. The prisms contain all of the materials heretofore employed in the porous cup, combined with others not before used, compressed into compact and convenient form by powerful hydraulic machinery.
When the elements have become exhausted from long service the prisms should be taken off, new prisms should be attached, and the battery set up as before, with new zinc and fresh sal-ammoniac.
Only pure sal-ammoniac and well amalgamated zinc rods should be used.
CHAPTER XVI.
The Diamond Carbon Battery.
This cell, Fig. 12, is similar in action to the Leclanche cell. Instead of using binoxide of manganese to reduce
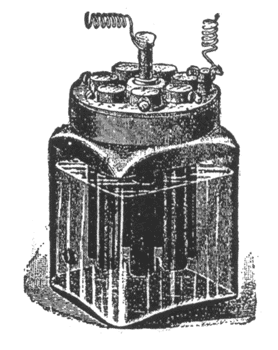 |
| Fig. 12. — Diamond Carbon Battery. |
polarization the surface of the carbon electrode is greatly increased, and the zinc electrode kept very small. Care must be taken to slip the small rubber ring over the lower part of the zinc rod to prevent contact between zinc and carbon. The cell is set up in the same manner as the Leclanche cell.
The inside of the rim of the jar is paraffined to prevent creeping of salts, and the cover fits very tightly to reduce evaporation.
CHAPTER XVII.
Gravity Batteries.
Gravity batteries belong to the class of two-fluid cells.
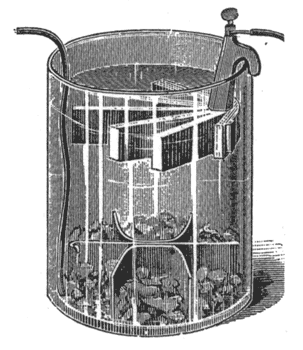 |
| Fig. 13. — Gravity Battery. |
Figs. 13 and 14 show two different styles, which are recognized as the leading representatives of the gravity type. Instead of employing a porous cell to keep the two liquids separate, it is possible, where one of the liquids is heavier than the other, to keep the latter on the bottom, and have the lighter floating upon it; this separation, however, is never perfect, the heavy liquid slowly diffusing upward. These batteries are set up as follows:
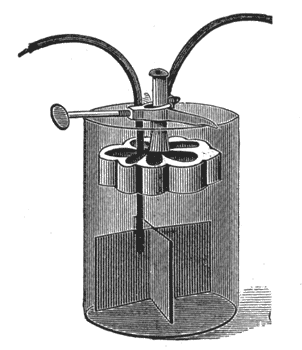 |
| Fig. 14. — Gravity Battery With Adjustable Hanger. |
Unfold the copper strip and place it in the bottom of the jar. Suspend the zinc about four inches above the copper. Pour clean water into the jar so as to cover the zinc. Then drop in blue vitriol in small lumps, not over six or eight ounces per cup at one time.
The resistance may be reduced and the battery be made immediately available by drawing about half a pint of solution of sulphate of zinc from a battery already in use, and pouring it into the jar; or, when this cannot be done, by putting into the liquid four or five ounces of pulverized sulphate of zinc.
Blue vitriol should be dropped into the jar as it is consumed, care being taken that it goes to the bottom. The need of blue vitriol is shown by the fading of the blue color, which should be kept as high as the top of the copper, but should never reach the zinc.
After the battery has been started no further attention is required, except to keep it supplied with blue vitriol, until the quantity of sulphate of zinc in solution has become too great. In that case draw out a portion of the top of the liquid with a syringe or a cup, and replace it with clear water.
As long as the battery continues in action there is an increase of the quantity of sulphate of zinc in solution in the upper part of the jar.
A hydrometer is convenient for the purpose of testing the strength of this solution. When the specific gravity is less than 15 degrees, there is too little sulphate of zinc; when it is 35 degrees or over, there is too much in solution, and it must be diluted.
When the zincs become coated so as to interfere with the action of the battery they must be taken out and scraped clean and washed.
CHAPTER XVIII.
Dry Batteries.
Very often it is necessary to arrange batteries so that they may stand considerable jarring, or even overturning. A liquid, of course, would be spilled. Dry cells, for instance, are needed in railroad cars for the electric bells, in military telegraphy, and in portable testing sets. One of the early batteries of this type is Sir Wm. Thomson's sawdust battery, which is, in fact, a Daniell cell filled with sawdust.
 |
| Fig. 15. — Dry Battery. |
Gassner's battery, Fig. 15, is considered one of the best dry batteries, and is extensively used in places where a cell containing a fluid would be objectionable. This element is mounted in a strong zinc case. The top of the battery is hermetically closed, and, consequently, no crystallization of salts takes place at the surface. Batteries are furnished ready for use, and will work well until completely exhausted. They can be used with great advantage in places subject to high or low temperature. When at rest no chemical action takes place; the component parts of the battery are consequently not consumed. These desirable properties of the dry battery are due in the first place to the peculiar porous substance contained in the battery in place of the solutions which, although it forms a compact or nearly solid mass, allows the gases to pass. The porous mass forms no deposit on the zinc which would tend to increase the internal resistance, and consequently, weaken the current. The necessary moisture is contained in the battery in chemical combination, and not in form of a free liquid.
CHAPTER XIX.
Classification of Batteries.
Batteries may be classified, according to their use, into open circuit and closed circuit batteries.
Open Circuit Batteries. — These batteries are used where a current is needed for a few seconds at a time only. If the circuit is kept closed for any length of time, these batteries will become rapidly polarized, and the current will be so weak as to be unable to do the work. When the circuit is opened the battery will recover itself in time. These batteries are applicable to bells, alarms, telephones, gas-lighters, etc. To this class belong the Leclanche, diamond carbon, Gassner and Smee.
Closed Circuit Batteries. — These are used for continuous work, as, for Instance, electro plating, charging accumulators, electric lighting, etc., or when a current is required to flow continuously through a wire, and an apparatus so arranged as to signal the break of this wire, as in certain safe-protecting devices or police call boxes. This class of batteries may be subdivided into:
 |
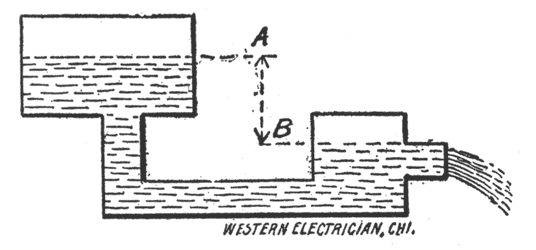
| Fig. 16. — Reservoirs of Water. |
A. Batteries with a constant current, able to do considerable work, such as electro plating, electric lighting, running motors, etc., for hours: at a time.
B. Batteries with a constant current, able to do very little work, such as in safe-protecting devices, several medical appliances and alarms, but able to do this continuously for months without any attention.
To class A belong Grenet, Grove, Bunsen, carbon, nickel-plating and Fuller.
To class B belong Daniell and gravity.
ELEMENTARY DATA.
CHAPTER XX.
The Electric Current.
The reader has now learned how the electric current is practically generated in voltaic or galvanic cells or batteries.
In order to understand the action of what is called the electric current, we will consider two. reservoirs of water connected by a pipe, Fig. 16. The electric current may be likened to the flow of water through this pipe from the higher to the lower level.
The unit of current strength, also called the rate of flow, or intensity, is the ampere. In the illustration we would say the water is flowing through the pipe at the rate of one gallon per second. In speaking of the electric current we would say it has a strength of, say, one ampere.
The unit of electromotive force, also called electrical pressure, or tension, or difference of potential, is the volt. In the illustration the head of water, or the difference between the levels, A and B, is similar to the electromotive force.
The unit of resistance is the ohm. Resistance may be compared with the friction of the internal surface of the pipe offered to the water. It follows that the more cross-section the less friction, and the less cross-section the more friction, for the same volume of water flowing through the pipe in a given time.
In the following only the terms, current strength, electro-motive force — frequently written e.m.f. — and resistance will be used.
CHAPTER XXI.
Ohm's Law.
Ohm's law expresses the relation of current strength, electromotive force and resistance to each other. It says: Current strength equals electromotive force divided by resistance. We can write this C = E/R, C standing for current strength, E for electromotive force, and R for resistance.
CHAPTER XXII.
Conductors and Insulators.
Bodies in which the current moves freely are called conductors, and those in which it does not move freely are called insulators. Examples of conductors: Metals, solutions of chemical salts, moist earth, etc. Examples of insulators: Porcelain, rubber, gutta-percha, sealing wax, dry wood, etc.
CHAPTER XXIII.
Direction of the Current.
For convenience sake we assumed that the electric current flows from the positive pole of a galvanic cell through the external circuit back to the negative pole. In order to find the direction in which the
 |
| Fig. 17. — Pocket Compass. |
current is passing through the wire, we place a pocket compass, Fig. 17, under the wire, Fig. 18. The north-seeking pole of the needle, called the north pole, and distinguished either by a different color — blue, for instance — or by a little brass rivet, will be deflected to the left when the current flows in the direction of the arrow, Fig. 18. Or, in other words, the positive electricity enters at A and leaves at B.
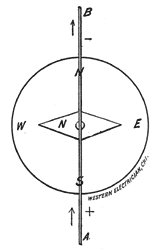 |
| Fig. 18. — Compass Under the Wire. |
When the compass is held above the wire the north pole of the needle will be deflected to the right.
General rule: Imagine yourself swimming in the current, always facing the needle. The positive current entering at your feet will cause the needle to deflect to your left.
Another general rule: Think of the word Snow. With the wire over the compass the current flowing from the South to the North will deflect the needle to the West; in other words, from S-outh to N-orth O-ver W-est.
The compass should be tested before these trials are made, as the polarity may have been reversed by the proximity of dynamo electric machines or other powerful magnets.
(To be Continued.)
BELL HANGERS' HAND-BOOK, by F. B. Badt; in course of preparation; about 100 pages, 80 illustrations; flexible cloth binding; type page 6 inches by 3 inches; price $1. All rights reserved.
