[Trade Journal]
Publication: Western Electrician
Chicago, IL, United States
vol. 3, no. 15, p. 194-195, col. 2-3,1-3
Bell Hangers' Hand-Book.
By F. B. BADT.
PART I.
VOLTAIC ELECTRICITY.
CHAPTER I.
Contact Series.
Two dissimilar metals brought in contact produce opposite kinds of electricity on the two surfaces, one becoming positively (+) and the other negatively ( -) electrified. In the following table metals are arranged in such a series that each becomes positively electrified when placed in contact with one below it:
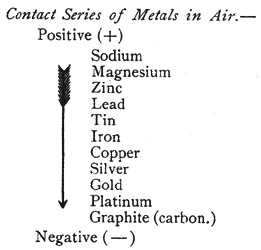 |
CHAPTER II.
The Voltaic or Galvanic Cell.
To make a simple voltaic or galvanic cell, place in a glass jar water, acidulated with a few drops of sulphuric acid, a strip of zinc, and a strip of copper. This cell is capable of generating a continuous flow of electricity through a wire, whose ends are connected to the two metal strips. See Fig. I.
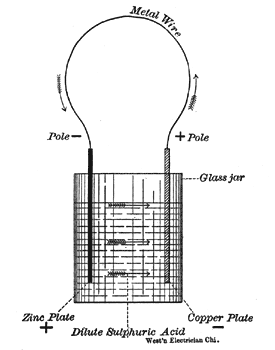 |
| Fig. 1. Simple Voltaic or Galvanic Cell. |
CHAPTER III.
Generation of Current.
The current in such a cell, as shown in Fig. 1, starts from the positive zinc plate, flows through the fluid to the copper plate, out through the external circuit and back to the zinc plate. The copper strip, whence the current starts on its journey through the external circuit, is called the positive pole (+) and the zinc strip is called the negative pole (—). When the external circuit is broken no current flows, but still the wire connected to the positive pole (copper plate) is called the positive wire, and the wire connected to the zinc pole the negative wire. As in almost all commercial batteries zinc is used as one pole, it may be well to remember that the zinc pole is always the negative pole.
When the current flows the zinc strip is observed to waste away. Its consumption furnishes the energy required to drive the current through the fluid from zinc to copper, and through the external circuit. At the same time it will be noticed that a few bubbles of hydrogen gas appear on the surface of the copper plate. Both these actions go on as long as the wires are joined to form a complete circuit. Thus the production of an electric current by a voltaic cell is always, accompanied by chemical action in the cell. Zinc and the other metals which stand at the electro-positive end of the contact-series will be dissolved, while the electro-negative substances — copper, silver, gold, platinum and graphite — will not be attacked.
A piece of quite pure zinc when dipped, alone into dilute sulphuric acid is not attacked by the liquid. The ordinary commercial zinc, however, is not pure and will be dissolved, a large quantity of hydrogen bubbles being given off from the surface of the metal.
As shown before, when the current flows through the cell and chemical action commences, the bubbles of hydrogen are evolved not at the zinc plate, nor throughout the liquid, but at the surface of the copper plate. This apparent transfer of the hydrogen gas through the liquid from the zinc to the surface of the copper plate must be borne in mind to understand the action of the different voltaic cells.
CHAPTER IV.
Local Action.
When the circuit is not closed, the current cannot flow and there should be no chemical action. The impure zinc of commerce, however, will continuously dissolve in the acid and give off hydrogen bubbles. This is called local action. It is caused by impurities in the zinc, such as particles of iron or other metals which behave in contact with the particles of zinc and the acid like miniature voltaic cells, and thus cause a constant waste of the zinc even if the battery circuit should be open.
To do away with this local action the zinc plates are amalgamated. The iron particles do not dissolve in the mercury, but are carried off from the surface of the zinc plate by the hydrogen bubbles. As the zinc in the amalgam dissolves the film of mercury unites with fresh portions of zinc, and consequently always presents a clean, bright surface to the liquid. The amalgamation of the zinc plates may be very well done by first immersing the zincs in a solution of dilute sulphuric acid and then in a bath of mercury. A brush or cloth may be used to rub them, so as to reach all points of the surface. Where a large number of zincs is to be amalgamated, the following will be found to be a good method: Dissolve eight ounces of mercury in a mixture consisting of two pounds of hydrochloric and one pound of nitric acid; when the solution is complete, add three pounds of hydrochloric acid. The zincs are amalgamated by immersing them in this solution for a few seconds; they should then be removed to a vat of clear water and rubbed as in the first case with a brush or cloth. If the solution is kept in a covered vessel it may be used a number of times.
CHAPTER V.
Polarization.
The bubbles of hydrogen liberated at the surface of the copper electrode stick to it in great numbers, and form a film over its surface; hence the effective amount of service of the plate is very much diminished in a short time. This will cause an immediate falling off in the strength of the current, sometimes even stopping it entirely. A battery in this condition is said to be polarized. The effects of polarization are: First, it weakens the current by the increased resistance which it offers to the flow, for bubbles of gas are bad conductors; and secondly it weakens the current by setting up an opposing electromotive force, for hydrogen is nearly as oxydizable as zinc, and is electro positive.
It is, of course, very important to prevent this polarization, as otherwise the current would not be constant.
Various remedies are employed. These may be classed as mechanical, chemical and electro chemical.
1. Mechanical Means. — The liquid may be agitated or air may be blown through it, thus preventing the hydrogen bubbles from sticking to the positive pole.
The surface of the latter may be roughened so the bubbles will collect at the points and be quickly carried off. Example: Smee's cell.
2. Chemical Means. — If a highly oxidizing substance be added to the acid, it will prevent the formation of hydrogen bubbles, as the oxygen of this substance will combine with the hydrogen and from water. Such substances are bichromate of potash, binoxide of manganese, nitric acid, and chloride of lime. These substances, however, would attack the copper, and they can only be used in zinc-carbon or zinc-platinum cells. Nitric acid also attacks zinc when the circuit is open and cannot be employed in the same single cell with the zinc plate. Examples of cells: Grenet, Grove, Bunsen, carbon, nickel plating, Fuller, Leclanche, diamond carbon.
3. Electro Chemical Means. — Double cells can be arranged in such manner that a solid metal such as copper shall be liberated instead of hydrogen bubbles. This entirely prevents polarization. Examples of cells: Daniell, gravity.
A battery to be really good should fulfill the following conditions:
Its electromotive force should be high and constant.
Its internal resistance should be small.
It should give a constant current and must therefore be free from polarization, and not liable to rapid exhaustion requiring frequent renewal of material.
It should consume no material when the circuit is open.
It should be cheap, and of durable materials.
It should be manageable and if possible, should not emit corrosive fumes.
No single battery fulfills all these conditions, however; some batteries are better for one purpose and some for another. Thus, for telegraphing through a long line of wire, a considerable internal resistance is of no great consequence, as it is but a small fraction of the total resistance in circuit.
For electric gas lighting or other low resistance circuits, on the other hand, much internal resistance would be, if not absolutely fatal, certainly a positive disadvantage.
CHAPTER VI.
Classification of Cells.
According to the foregoing voltaic cells may be classified as single fluid and two fluid cells or batteries. Following are descriptions of the leading representatives of these two classes:
SINGLE FLUID BATTERIES.
CHAPTER VII.
The Smee Battery.
This cell Fig. 2, is an improvement on the simple volta zinc-copper cell; it consists of two zinc plates, forming one ploe [sic] pole, and one platinized silver plate the other pole, both dipping into dilute sulphuric acid. The polarization of this one-fluid battery is avoided by rough-coating the silver plate with finely divided platinum, which liberates the hydrogen bubbles freely; nevertheless, the current would fall off greatly after closing the battery for a few minutes. This battery is charged with a solution of one part sulphuric acid to seven of water. The plates are connected to the clamp and placed in the jar. In this battery, above all the precaution of amalgamating the zinc should never be neglected. With an unamalgamated zinc the results are very unsatisfactory.
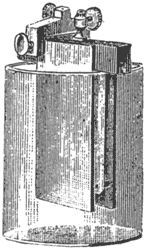 |
| Fig. 2. — Smee Battery. |
CHAPTER VIII.
The Grenet Battery.
The glass jar of this cell is generally made in the shape of a bottle. A well amalgamated plate of zinc forms one pole, and a pair of carbon plates, one on each side of the zinc, joined at the hard rubber top, forms the other pole. In Fig. 3, the carbon plates are marked C and the zinc plate Z. The bottle is filled with bichromate of potash and dilute sulphuric acid. [See electropoion fluid.] As this solution acts on the zinc when the circuit is open the zinc plate is fixed to a brass rod, by which it can be drawn up out of the solution when the cell is not in use. It is almost the only single-fluid cell free from polarization, and even in this form the strength of the current falls off after a few minutes' use, owing to the chemical reduction of the liquid.
This battery is especially adapted for experimental and illustrative purposes. It occupies but little space, furnishes a great quantity of current, and, as the zinc can be raised from the fluid, may be kept charged, ready for use, for many months, and can be set in action any time when required by simply depressing the brass rod which slides through the center of the cover of the cell, and to which the zinc is attached. For operating induction coils, which are frequently used for electric gas lighting, and for heating effects in medical practice, it is unequaled. The battery is charged by pouring electropoion fluid into the cell until it reaches nearly to the top of the globular part, and then drawing up the zinc and placing the element in the cell The fluid should not be so high as to touch the zinc when the latter is drawn up.
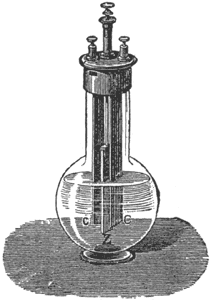 |
| Fig. 3. — Grenet Battery. |
Electropoion Fluid. — Recipe for electropoion fluid: Mix well 100 parts of water, 12 of bichromate of potassium, and 25 of sulphuric acid. The last should be carefully added, as great heat is evolved in the operation.
TWO-FLUID BATTERIES. .
CHAPTER IX.
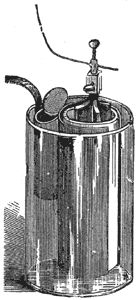
The Daniell Battery.
Each cell or element consists of a glass vessel with an inner and an outer cell, divided by a porous partition to keep the separate liquids in the two cells from mixing, Fig. 4. A copper cylinder is placed outside and a rod of amalgamated zinc inside the porous cup. The liquid in the inner cell is dilute sulphuric acid; that in the outer cell a saturated solution of blue vitriol (sulphate of copper). Some crystals of the same substance are placed in a perforated pocket at the top of the cell in order that they may dissolve, and replace that which is used while the battery is in action. When the circuit is closed
 |
| Fig. 4. — Daniell Battery. |
the zinc dissolves in the dilute sulphuric acid, and liberates hydrogen gas. The gas, however, does not in this case appear in bubbles on the surface of the copper electrode, as, by a chemical process, the freed atoms of hydrogen traverse the pores of the porous cup, and are exchanged for copper atoms. So while the zinc dissolves the copper grows, the dilute sulphuric acid gradually changing into sulphate of zinc, and the sulphate of copper into sulphuric acid. There is, therefore, no polarization so long as the copper solution is saturated and the battery is very constant. It is mainly used as a standard cell for electrical measurements and in telegraphy.
The directions for gravity battery, given later on, will apply to the maintenance of the Daniell.
CHAPTER X.
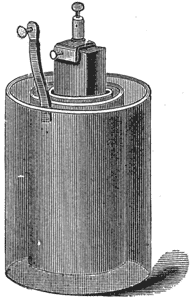
The Grove Battery.
This battery, Fig. 5, consists of a glass or ebonite jar containing the amalgamated zinc cylinder and dilute sulphuric acid in the inner porous cup a piece of platinum foil dips into nitric acid of the greatest strength. There is no polarization, for the hydrogen liberated at the zinc plate, in passing through the nitric acid on its way to the platinum pole, decomposes the nitric acid, and is itself oxidized, producing
 |
water and the red fumes of nitric peroxide gas. This gas does not produce polarization, as it is readily soluble in nitric acid. The battery has both greater electromotive force and lower internal resistance than the Daniell, and will furnish continuously for three or four hours a strong current.
Directions for setting up: The glass jar is filled with dilute sulphuric acid, about one part of acid to twenty of water, and the porous cup, with fuming nitric acid, about 40°. The platinum plate being placed in the porous cup and the zinc in the glass jar, the battery is ready for use. The plates should be removed and cleaned, and the nitric acid emptied out when the battery is not in use. The binding screws should be examined, and the zincs amalgamated before again setting up the battery.
The fumes of this battery are poisonous, and should not be inhaled.
CHAPTER XI.
The Bunsen Battery.
This battery, Fig 6, is a modification of the Grove. The expensive platinum foil is replaced by a plate of hard gas carbon.
 |
| Fig. 6. — Bunsen Battery. |
The battery is set up in the same manner as the Grove.
Care must be taken to get a good clean contact between the carbon and the brass clamp.
CHAPTER XII.
The Carbon Battery.
The carbon battery, Fig. 7, is identical with the Bunsen.
 |
| Fig. 7. — Carbon Battery. |
Diluted sulphuric acid is used in the outer jar, and electropoion fluid, instead of nitric acid, in the porous cups.
CHAPTER XIII.
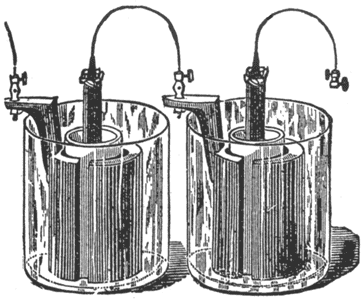
Nickel-Plating Battery.
This battery, Fig. 8, also is a modification of the Bunsen cell. It is of great power, and is used for nickel and electroplating, running electric motors, etc.
 |
| Fig. 8. — Nickel Plating Battery. |
In setting up the battery amalgamate the zincs thoroughly, inside and out. Into each porous cup put two ounces of nitric acid, and half fill the cup with a mixture of equal parts, by measure, of water and sulphuric acid Place the carbon in the porous cup, and add the mixture until it reaches the proper height, as mentioned below. Put the zinc in the outer or glass jar, and fill to the top of the zinc with a mixture of one part of sulphuric acid to twelve parts of water, previously mixed and allowed to cool. The fluids in the porous cup and outer jar should be of the same height. When the liquid in the jar becomes milky replace it with fresh solution. Add occasionally a little nitric acid to the liquid in the porous cells, and keep the zincs well amalgamated.
Instead of nitric acid electropoion fluid may be used in the porous cup.
CHAPTER XIV.
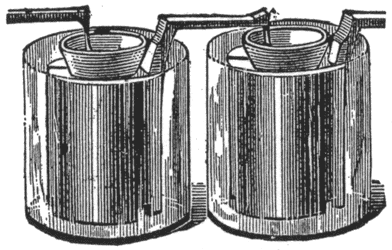
Fuller's Mercury Bichromate Battery.
A solid, well amalgamated block of zinc put in the porous cup forms one pole; a carbon plate in the outer jar the other pole. See Fig. 9.
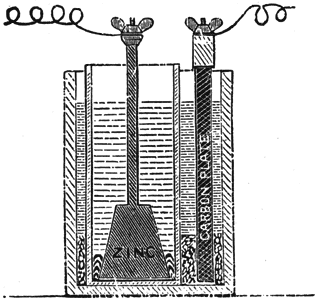 |
| Fig. 9. — Fuller's Mercury Bichromate Battery. |
This cell is set up as follows: Make a paste by mixing pulverized bichromate of potash with strong sulphuric acid in about equal parts by weight. Put about ten ounces of this paste into the outside jar, pour over it two or three ounces of sulphuric acid, and fill with water. Into the porous cell pour a tablespoonful of mercury, put the zinc in place, and fill with water. The zinc should be lifted out occasionally, and the sulphate washed off. Keep a supply of mercury in the porous cell, so as to have the zinc always well amalgamated.
This cell is largely used in telegraphy, especially in England. It will stand longer continuous use than the Leclanche, but it is not so economical.
BELL HANGERS' HAND-BOOK, by F. B. Badt; in course of preparation; about 100 pages, 80 illustrations; flexible cloth binding; type page 6 inches by 3 inches; price $1. All rights reserved.
