[Trade Journal]
Publication: Western Electrician
Chicago, IL, United States
vol. 22, no. 12, p. 175-176, col. 3,1
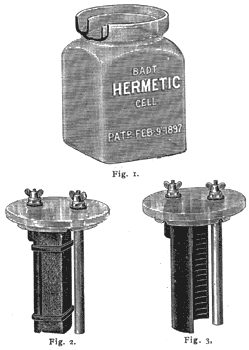
Badt Hermetic Cell.
The accompanying cuts illustrate the Badt hermetic cell which has only recently been placed upon the market, but which has been the object of much interest among primary-battery men who have been acquainted with the experiments conducted by Lieutenant F. B. Badt in perfecting it. It is an air-tight open-circuit cell with oil seal and with oil insulation. The claims for this new cell are strikingly presented in the following list of negative qualities: No evaporation, no creeping of salts, no leakage of electricity, no premature disintegration of zincs, no trouble of any kind, no attention, no attendance, no repair bills. Finally, it is claimed that while it is "expensive in first cost" it is "cheap in the long run."
 |
| Badt Hermetic Cell. |
The manufacturers of. the Badt hermetic cell direct attention to many of the failings common to cells of this class which it is claimed are overcome in the Badt cell. All who have had experience in handling primary batteries realize the annoyance caused by evaporation of the liquid and the creeping of salts. This creeping of salts is usually started by the evaporation of the liquid. If there is no creeping of salt, of course there will be no danger from short-circuiting of the electrodes within the cell.
The advantage gained by maintaining a uniform level of the solution is easily seen by observing a wooden post driven into the river. The wood will rot there where it is exposed alternately to the air and to the water, namely, near the surface of the water. A similar action takes place in an electric cell where the zinc often is found eaten through and used up only where the gradual sinking of the level of the solution has exposed portion's to the influence of various chemical and electro-chemical agencies. Experience shows that with the surface of the solution kept at the same level, this zinc-destroying effect will not take place. The oil seal absolutely insulates the glass cover from the jar, and as no salt can creep over the oil, the cover with its electrodes may be said to be insulated by means of an oil insulator from the jar and from earth. The cover, being of glass, insulates the electrodes from each other.
Fig. 1 represents the glass jar without cover, showing a part broken away, disclosing the channel or trough surrounding the upper edge of the jar. This trough in practice is filled with oil. Fig. 2 represents the glass cover with electrodes of cell No. 1 (hermetic prism cell). The cover is provided with two rims or flanges. The inner flange rests in the oil trough and the outer flange surrounds the top of the jar and prevents dust from getting into the oil trough. There are three notches at the lower edge of the inner flange to prevent the displacement of the oil in the trough by either internal or external pressure due to the electro-chemical action in the cell. Each electrode (zinc and carbon) has a shoulder carrying a metal terminal which passes through a hole of the cover, and soft rubber washers are placed on these terminals below and above the glass, in order to make the holes air-tight. Fig. 3 represents the glass cover with electrodes of Cell No. 2 (hermetic cell without prism or chemical depolarizer). The glass cover is identical with that for cell No. 1, so is the zinc; the carbon, however, is of a peculiar shape and has no prism (chemical depolarizer) attached to it.
This cell is placed on the market by Meysenburg & Badt of Chicago.