[Trade Journal]
Publication: Electrical Review
New York, NY, United States
vol. 6, no. 18, p. 3, col. 1
The Grenet and Fuller Mercury
Bichromate Batteries.
We illustrate on this page three forms of battery manufactured by the well-known house of J. H Bunnell & Co. The Fuller mercury bichromate battery, Fig. 1, is a most excellent one for any or all of the uses to which strong carbon batteries are adapted. Having all the powerful qualities of any of the various forms of carbon-electropoion batteries, its two great advantages consist in its being self-amalgamating from the supply of mercury in the porous cell, and in the great amount of sulphuric acid and bichromate (electropoion) capable of being held in the outside jar, thus giving much longer action than can be sustained in other acid batteries. At the same time its quantitative effects may be very greatly increased by doubling or trebling the carbon surface, in other words, using two or three carbons instead of one. By this change the Fuller battery is made to be one of the very best and cheapest forms of electro-plating battery that it is possible to construct. As this battery is capable oĢ remaining on open circuit for weeks at a time without much deterioration, and without any attention, it is well adapted for ringing very large electric bells, or for any use where a specially powerful open circuit battery is required. This form is also well adapted for a laboratory test battery, and for all general experimenting.
 |
| Fig. 1. the New Fuller Mercury-Bichromate Battery. |
This battery is strongly endorsed by Prof. Farmer and other leading electricians, as one of the strongest and most durable batteries ever introduced, and is unsurpassed for the purposes for which it is specially designed.
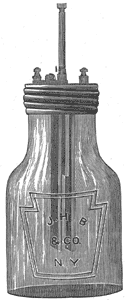
The very frequent inquiry for batteries which are sealed, to prevent spilling or evaporation, has induced Bunnell & Co. to put together the Fuller battery in the above shape, Fig. 2. A hard rubber top, having under it a sheet of gum packing, is clamped tightly to the jar by means of a screw shell of sheet metal made to engage the threaded top of the glass jar and clamp down the rubber cover in its place. Into the hard rubber top is inserted the porous cup, into the mouth of which is placed a rubber cork carrying a zinc. The carbon plate adjoining the porous cup is also permanently fastened to the hard rubber top. In this way the complete element is in a very practical shape, readily taken apart to clean or fill, and equally readily sealed tightly and safely.
 |
| Fig. 2. the Fuller Mercury-Bichromate Battery. |
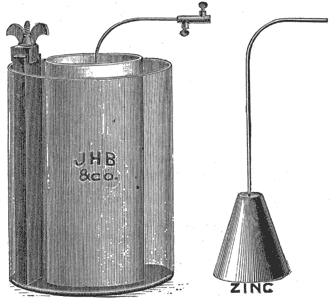
Figure 3 illustrates the new American form of the Grenet battery. The large cut represents a cell of improved form of Grenet battery. It consists essentially of two carbons, with a zinc plate between them. It is similar in construction to the French pattern, and will produce as strong a current as the 10-inch cell of that make, while its cost is less. When the battery is not in use the zinc is saved by being raised from the liquid. The battery may remain in this condition for a long time without attention, and may be placed in service at any moment by depressing the brass rod which carries the zinc.
 |
| Fig. 3. the Grenet Battery (American Form). |
