[Trade Journal]
Publication: Electrical World
New York, NY, United States
vol. 4, no. 26, p. 268, col. 2
The Orne Battery.
For a long time electricians have been experimenting to obtain a battery, using an acid liquid for an excitant, in which there would be no chemical action when the circuit is open. Among recent improvements in electrical apparatus of this kind is the Orne battery, which is said to accomplish the purpose in a satisfactory manner. This battery is made by the Orne Battery Company, of 837 Main street, Cambridge, Mass., and is illustrated below. It is composed of a piece of carbon 4 inches long, 1 1/2 inches wide, 1/4 inch thick, and a porous cup 3 inches long and 1/2 inch in diameter, filled with a mixture of zinc and mercury. The carbon and cup are placed in a frame and suspended in a glass jar. The jar is filled with a solution of sulphuric acid and. bichromate potassa. The electromotive force is 2 volts; resistance, 3 ohms; current, .66 ampere. There is claimed to be no local action, thus giving the full value of zinc and acid in electrical work. It is clean, does not give off acid or
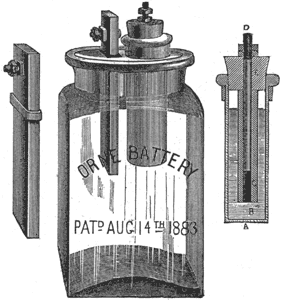 |
corrosive fumes, has no formation of crystals or evaporation of solution. The connections do not corrode. There is no consumption of zinc when the circuit is open. It will last from six months to two years before it has to be refilled. When the battery has to be renewed, there are no parts to throw away; all that has to be done is to refill the jar with acid solution, and place a piece of zinc in the porous cup. Every part is movable. The same amount of surface of amalgam or zinc and mercury is always presented to the acid. If the circuit gets accidentally closed, the battery is so constructed that it runs only five minutes and then stops working, thus saving waste of zinc and acid and the use of automatic cut-outs. It can be brought back to its original strength immediately by rinsing the porous cup with water. This action is due to the rapid formation of dry sulphate of zinc on the inside of the porous cup, thus separating the amalgam of zinc and mercury from contact with the solution.
It should be stated that any solution can be used with this battery; but a solution made as above gives the strongest current. The level of the mercury amalgam being above the level of the solution, the latter is prevented from flowing through the walls of the cup and dissolving the zinc when the battery is at rest. In the cut, A is the porous cup, B the amalgam of zinc and mercury, C the hollow rod of zinc, D the brass rod, and E the stopple.
