[Trade Journal]
Publication: Electrical World
New York, NY, United States
vol. 8, no. 15, p. 176, col. 1-3
The Roberts Peroxide Battery.
BY I. L. ROBERTS.
In a previous article in THE ELECTRICAL WORLD* I have stated that no galvanic action can take place unless water be present. This is true so far as our knowledge of electrical measurements or electrical phenomena reaches.
It is probable, however, that when coal is consumed in oxygen or a metal is burned in chlorine or fluorine, the action is electric; or, in other words, all chemical action first produces a current of electricity. But as we do not know how to attach binding posts to such elements, the current is not made manifest to our senses. As the currents thus made are entirely short-circuited on themselves, they produce only the phenomena of heat, as does the current in an ordinary battery when short-circuited on itself. As soon as we are able to get some way of conducting away the current from simple chemical union, then we will reach the ideal method of producing electricity.
At present, we know of no other way of producing galvanic currents than by the use of acid radicals through the agency of water. Thus, if sulphuric acid, H2SO4, be mixed with twenty times its weight of water and placed in a glass jar containing a strip of well-amalgamated zinc and a strip of platinum, no action takes place. But if the platinum be brought in contact with the zinc, a great shower of gas bubbles will begin to rise immediately from the surface of the platinum. These bubbles of gas, if collected and examined, will be found to be pure hydrogen. The sulphuric acid radical SO4 has taken one atom of zinc and formed the salt ZnSO4, zinc sulphate, and given up its hydrogen. It is this element of hydrogen that forms the subject of the present article.
If a galvanometer is placed in the circuit of the simple battery above described, the needle will at first indicate a certain current which will gradually fall nearer and nearer to zero, not because there is an insufficiency of acid or zinc, but because this hydrogen gas, when divorced from its radical, SO4, is in the nascent state for an instant, and has the power of partially uniting or clinging on to any conductor yet discovered. It has the least affinity for platinum or carbon of all known substances. If allowed to remain untouched, the electrodes will in a few minutes lose fully half their power, becoming coated or saturated with hydrogen, and are then in the condition known as polarized. Just here is the rock on which the hopes of many inventors in electrodynamics have come to grief. There have been many mechanical devices tried, such as shaking, rotating the elements, blowing currents of air against the plates, and the like, but none of them have been successful to a satisfactory degree, so as to warrant their general introduction, for the simple reason that there is a deeper fact which must be viewed from a new stand-point.
This fact is, that hydrogen in sulphuric, acid has a certain affinity for its radical, SO4, as well as for zinc. The proportion is easily found by taking a platinum or carbon conductor, together with a large piece of well-amalgamated zinc, and short-circulating them for, say, four hours in a large vessel filled with sulphuric acid and water, so that the acid will not be exhausted. At the end of t.hat time the platinum will have absorbed all the hydrogen it is capable of, and if the circuit is quickly broken and measured, the absolute electromotive force of zinc over hydrogen will be noted. It will be surprisingly small, about 1/2 volt. The same may be tried with chlorine in hydrochloric acid, HCI, or iodine or fluorine, or any acid, even vinegar (acetic acid). They all give different electromotive forces in proportion to the affinity of their radicals for hydrogen.
The hydrogen can be displaced in one of these acids by a metal, thug forming a salt, and this salt may be used as the electrolyte.
This, however, may be said to be going from bad to worse, as the metal of the radical adheres to the zinc still more tenaciously, and completely coats it unless kept separate by a porous cup, which is a poor preventive. But this is not the case with metallic bases more electropositive than zinc, such as all the alkalies, but the difficulty with the hydrogen is not at all removed if an alkaline salt is used, such, for instance, as sal-ammoniac, NH4Cl, or table salt, NaCl. Here the chlorine will leave the sodium to unite with the zinc. But this action would not take place if the sodium could not also get something to unite with. Hence, it splits an atom of water, and makes NaHO + H, sodium hydroxide and free hydrogen. In the case of sal-ammoniac, Zn + NH4Cl = ZnCl + NH3 + H. Here, as before, is the omnipresent hydrogen. In the earlier days chemists attempted to absorb this gas instead of getting rid of it, and as might have been expected, it was not only absorbed, but the electromotive force of the battery was more than trebled. Now the reason of this is that, weight for weight, hydrogen when oxidized yields more than four times as much energy as carbon and more than twenty-five times as much as zinc.
Poggendorff, Bunsen and Grove were the first chemists to make such attempts with the success that all know. Poggendorff used bichromate of potassium, and Bunsen and Grove, strong nitric acid.
These substances were used because they contained large amounts o( oxygen, which would unite with hydrogen. They were used in contact with the carbon or platinum, and absorbed the hydrogen as fast as the zinc took up the acid radical, yielding in electric energy the equivalent of its heat energy.
These batteries were the greatest electric generators in their day. But their cost and the great difficulty of keeping them in order, to say nothing of the crystallization with the Poggendorff method, and the fumes of the others, has rendered them nothing short of a nuisance. The recent discovery of methods of taking away the alkali from bichromate of soda and its great cheapness has given new life to the Poggendorff battery. Thus pure chromic acid can be purchased at the low price of 18 cents per pound in quantity. This is in the solid form, so that if the other difficulty of local action could be overcome, comparatively cheap electricity could be generated with it, using sulphuric acid and zinc
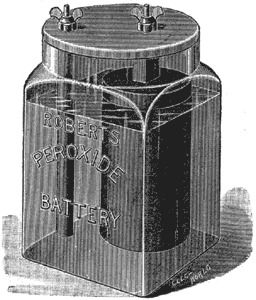 |
| Fig. 1. — the Roberts Peroxide Battery. |
The ideal battery is one which will not deteriorate on open circuit and will be constant on closed circuit. Both these qualities have been attained, but not in the same cell. Chromic acid as a depolarizer is constant enough; while sal-ammoniac, as a single fluid, will not destroy on open circuit to an injurious extent. I think I may venture to say that the nearest approach to the ideal battery that has come before the public is one recently invented by myself and known as my " Roberts' Peroxide Battery." This battery is represented in perspective in Fig. 1, and the peroxide element employed is shown in Fig. 2.
 |
| Fig. 2. — the Peroxide Element. |
The main feature in this battery is the peroxide of lead which is used as a depolarizer. It has been known in electro- chemistry for some time that peroxide of lead is one of the most powerful depolarizers, as used for instance in the secondary battery. But no commercial use has been made of it in primary batteries, because, first, it was a powder and difficult to apply to the purpose; and, secondly, it was too costly to put into competition with peroxide of manganese, which, though not so good, was cheap. It was the writer's fortune to discover how to make it cheap enough and solid enough to far outstrip manganese dioxide as a depolarizer.
This peroxide need not be of lead, but may be of silver, mercury or any easily oxidizable metal. It is made by adding to red lead, for instance, permanganate of potash in a powdered form and well mixing them. To this there is added any acid which will decompose the permanganate such as sulphuric, or preferably hydrochloric acid. This will unite with the alkali and liberate the permanganic acid, which is the most powerful oxidizer known to chemists. This will immediately unite with the red oxide of lead, Pb2O3, and produce PbO2. The whole mass, if properly made, is in a semi-liquid form, and if poured into a mold containing a carbon, Fig. 2, it will set in a few seconds. The mold can be opened, and the whole removed and dried, when it will be porous like a carbon and will be a conductor of electricity. It will be hard as carbon and will adhere firmly to the carbon core. This element can be used as a carbon in an acid battery with nothing but plain sulphuric acid and will furnish a constant current. For, as soon as the sulphuric acid radical acts on zinc and gives up its hydrogen.the following action takes place: Zn+H2SO4 = ZnSO4+H2. The liberated hydrogen unites immediately with an atom of oxygen, in the peroxide of lead and forms water, thus; H2 + PbO2 = PbO + H2O. Thus no polarization can take place as long as any peroxide of lead remains.
But it is with a solution of common table salt and a very little bichromate of soda that the nearest approach to the ideal battery is attained. There is then no local action, it being nearly constant, and the battery has an electromotive force of almost two volts. One of the ordinary size will give about three to four amperes. The bichromate of soda is added for the purpose of preventing the small amount of chloride of lead from dissolving and forming lead trees on the zinc.
If, however, a small quantity of it is added to the red lead mixture, it will produce the same result as the peroxide is formed. Chromic acid, if added to the red lead, will make a peroxide, but that will be inferior to the permanganic acid.
The element shown in the engraving will depolarize the hydrogen formed by the combustion of two ounces of zinc. This will yield about 46 ampere hours, with an average E. M. F. of 1.8 volts.
* See ELECTRICAL WORLD, Aug. 14.